CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells
- PMID: 16166380
- PMCID: PMC1221889
- DOI: 10.1101/gad.346205
CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells
Abstract
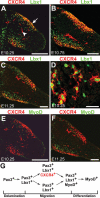
Long-range migrating progenitor cells generate hypaxial muscle, for instance the muscle of the limbs, hypoglossal cord, and diaphragm. We show here that migrating muscle progenitors express the chemokine receptor CXCR4. The corresponding ligand, SDF1, is expressed in limb and branchial arch mesenchyme; i.e., along the routes and at the targets of the migratory cells. Ectopic application of SDF1 in the chick limb attracts muscle progenitor cells. In CXCR4 mutant mice, the number of muscle progenitors that colonize the anlage of the tongue and the dorsal limb was reduced. Changes in the distribution of the muscle progenitor cells were accompanied by increased apoptosis, indicating that CXCR4 signals provide not only attractive cues but also control survival. Gab1 encodes an adaptor protein that transduces signals elicited by tyrosine kinase receptors, for instance the c-Met receptor, and plays a role in the migration of muscle progenitor cells. We found that CXCR4 and Gab1 interact genetically. For instance, muscle progenitors do not reach the anlage of the tongue in CXCR4;Gab1 double mutants; this target is colonized in either of the single mutants. Our analysis reveals a role of SDF1/CXCR4 signaling in the development of migrating muscle progenitors and shows that a threshold number of progenitor cells is required to generate muscle of appropriate size.
Figures






Similar articles
-
Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells.J Neurosci Res. 2004 Apr 1;76(1):20-34. doi: 10.1002/jnr.20001. J Neurosci Res. 2004. PMID: 15048927
-
A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors.Glia. 2005 May;50(3):258-69. doi: 10.1002/glia.20170. Glia. 2005. PMID: 15756692
-
A unique population of bone marrow cells migrates to skeletal muscle via hepatocyte growth factor/c-met axis.J Cell Sci. 2005 Oct 1;118(Pt 19):4343-52. doi: 10.1242/jcs.02555. Epub 2005 Sep 6. J Cell Sci. 2005. PMID: 16144866
-
Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice.Exp Hematol. 2006 Aug;34(8):967-75. doi: 10.1016/j.exphem.2006.04.002. Exp Hematol. 2006. PMID: 16863903 Review.
-
Recruitment of skeletal muscle progenitors to secondary sites: a role for CXCR4/SDF-1 signalling in skeletal muscle development.Results Probl Cell Differ. 2015;56:1-23. doi: 10.1007/978-3-662-44608-9_1. Results Probl Cell Differ. 2015. PMID: 25344664 Review.
Cited by
-
Gene expression profiling of skeletal myogenesis in human embryonic stem cells reveals a potential cascade of transcription factors regulating stages of myogenesis, including quiescent/activated satellite cell-like gene expression.PLoS One. 2019 Sep 27;14(9):e0222946. doi: 10.1371/journal.pone.0222946. eCollection 2019. PLoS One. 2019. PMID: 31560727 Free PMC article.
-
Chemokine expression and control of muscle cell migration during myogenesis.J Cell Sci. 2010 Sep 15;123(Pt 18):3052-60. doi: 10.1242/jcs.066241. Epub 2010 Aug 24. J Cell Sci. 2010. PMID: 20736301 Free PMC article.
-
Met and Cxcr4 cooperate to protect skeletal muscle stem cells against inflammation-induced damage during regeneration.Elife. 2021 Aug 5;10:e57356. doi: 10.7554/eLife.57356. Elife. 2021. PMID: 34350830 Free PMC article.
-
Neural Stem Cells for Early Ischemic Stroke.Int J Mol Sci. 2021 Jul 19;22(14):7703. doi: 10.3390/ijms22147703. Int J Mol Sci. 2021. PMID: 34299322 Free PMC article. Review.
-
Efficacy of Rectal Systemic Administration of Mesenchymal Stem Cells to Injury Sites via the CXCL12/CXCR4 Axis to Promote Regeneration in a Rabbit Skeletal Muscle Injury Model.Cells. 2023 Jun 27;12(13):1729. doi: 10.3390/cells12131729. Cells. 2023. PMID: 37443763 Free PMC article.
References
-
- Amthor H., Christ, B., and Patel, K. 1999. A molecular mechanism enabling continuous embryonic muscle growth—A balance between proliferation and differentiation. Development 126: 1041-1053. - PubMed
-
- Arnold H.H. and Braun, T. 2000. Genetics of muscle determination and development. Curr. Top. Dev. Biol. 48: 129-164. - PubMed
-
- Bagri A., Gurney, T., He, X., Zou, Y.R., Littman, D.R., Tessier-Lavigne, M., and Pleasure, S.J. 2002. The chemokine SDF1 regulates migration of dentate granule cells. Development 129: 4249-4260. - PubMed
-
- Birchmeier C., Birchmeier, W., Gherardi, E., and Vande Woude, G.F. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell. Biol. 4: 915-925. - PubMed
-
- Bladt F., Riethmacher, D., Isenmann, S., Aguzzi, A., and Birchmeier, C. 1995. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376: 768-771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
