Structural basis for the enhancement of eIF4A helicase activity by eIF4G
- PMID: 16166382
- PMCID: PMC1221891
- DOI: 10.1101/gad.1335305
Structural basis for the enhancement of eIF4A helicase activity by eIF4G
Abstract
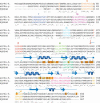
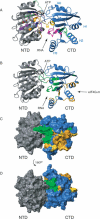
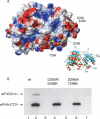
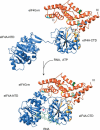
The eukaryotic translation initiation factors 4A (eIF4A) and 4G (eIF4G) are crucial for the assembly of the translationally active ribosome. Together with eIF4E, they form the eIF4F complex, which recruits the 40S subunit to the 5' cap of mRNA. The two-domain RNA helicase eIF4A is a very weak helicase by itself, but the activity is enhanced upon interaction with the scaffolding protein eIF4G. Here we show that, albeit both eIF4A domains play a role in binding the middle domain of eIF4G (eIF4G-m, amino acids 745-1003), the main interaction surface is located on the C-terminal domain. We use NMR spectroscopy to define the binding site and find that the contact surface is adjacent to the RNA-, ATP-, and eIF4A-NTD-interacting regions. Mutations of interface residues abrogated binding, confirmed the interface, and showed that the N-terminal end of eIF4G-m interacts with the C-terminal domain of eIF4A. The data suggest that eIF4G-m forms a soft clamp to stabilize the closed interdomain orientation of eIF4A. This model can explain the cooperativity between all binding partners of eIF4A (eIF4G, RNA, ATP) and stimulation of eIF4A activity in the eIF4F complex.
Figures

References
-
- Abramson R.D., Dever, T.E., and Merrick, W.C. 1988. Biochemical evidence supporting a mechanism for cap-independent and internal initiation of eukaryotic mRNA. J. Biol. Chem. 263: 6016-6019. - PubMed
-
- Ali I.K. and Jackson, R.J. 2001. The translation of capped mRNAs has an absolute requirement for the central domain of eIF4G but not for the cap-binding initiation factor eIF4E. Cold Spring Harb. Symp. Quant. Biol. 66: 377-387. - PubMed
-
- Bartels C., Xia, T., Billeter, M., Güntert, P., and Wüthrich, K. 1995. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR 5: 1-10. - PubMed
-
- Benz J., Trachsel, H., and Baumann, U. 1999. Crystal structure of the ATPase domain of translation initiation factor 4A from Saccharomyces cerevisiae: The prototype of the DEAD box protein family. Structure Fold. Des. 7: 671-679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
