Control of the expression and compartmentalization of (sigma)G activity during sporulation of Bacillus subtilis by regulators of (sigma)F and (sigma)E
- PMID: 16166546
- PMCID: PMC1251595
- DOI: 10.1128/JB.187.19.6832-6840.2005
Control of the expression and compartmentalization of (sigma)G activity during sporulation of Bacillus subtilis by regulators of (sigma)F and (sigma)E
Abstract
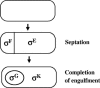
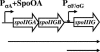
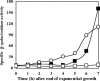
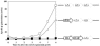
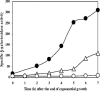
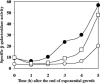
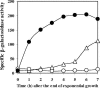
During formation of spores by Bacillus subtilis the RNA polymerase factor sigma(G) ordinarily becomes active during spore formation exclusively in the prespore upon completion of engulfment of the prespore by the mother cell. Formation and activation of sigma(G) ordinarily requires prior activity of sigma(F) in the prespore and sigma(E) in the mother cell. Here we report that in spoIIA mutants lacking both sigma(F) and the anti-sigma factor SpoIIAB and in which sigma(E) is not active, sigma(G) nevertheless becomes active. Further, its activity is largely confined to the mother cell. Thus, there is a switch in the location of sigma(G) activity from prespore to mother cell. Factors contributing to the mother cell location are inferred to be read-through of spoIIIG, the structural gene for sigma(G), from the upstream spoIIG locus and the absence of SpoIIAB, which can act in the mother cell as an anti-sigma factor to sigma(G). When the spoIIIG locus was moved away from spoIIG to the distal amyE locus, sigma(G) became active earlier in sporulation in spoIIA deletion mutants, and the sporulation septum was not formed, suggesting that premature sigma(G) activation can block septum formation. We report a previously unrecognized control in which SpoIIGA can prevent the appearance of sigma(G) activity, and pro-sigma(E) (but not sigma(E)) can counteract this effect of SpoIIGA. We find that in strains lacking sigma(F) and SpoIIAB and engineered to produce active sigma(E) in the mother cell without the need for SpoIIGA, sigma(G) also becomes active in the mother cell.
Figures



References
-
- Arabolaza, A. L., A. Nakamura, M. E. Pedrido, L. Martelotto, L. Orsario, and R. R. Grau. 2003. Characterization of a novel inhibitory feedback of the anti-anti-sigma SpoIIAA on Spo0A activation during development in Bacillus subtilis. Mol. Microbiol. 47:1251-1263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

