Cardioprotective role of the mitochondrial ATP-binding cassette protein 1
- PMID: 16166555
- PMCID: PMC2756018
- DOI: 10.1161/01.RES.0000186277.12336.11
Cardioprotective role of the mitochondrial ATP-binding cassette protein 1
Abstract
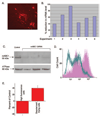
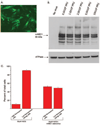
The mechanism by which mitochondria exert protection against oxidant stress is not clear. We recently showed that a purified mitochondrial fraction containing 5 coimmunoprecipitating proteins (succinate dehydrogenase, adenine nucleotide translocator, ATP synthase, inorganic phosphate carrier, and mitochondrial ATP-binding cassette protein 1 or mABC1) displayed mitochondrial ATP-sensitive K+-channel activity. mABC1, a member of the ABC family of proteins, is the only protein in this complex whose function is not known. A yeast homologue of mABC1 protein, Mdl1p, was recently identified to have a novel role for induction of cellular resistance to oxidant stress. Based on these observations, we hypothesized that mABC1 plays a key role in protection of myocardial cells against oxidant stress. We studied the function of mABC1 by modulating the levels of this protein in neonatal rat cardiomyocytes using various molecular techniques, followed by assessment of cell viability and measurement of mitochondrial membrane potential. RNA interference resulted in reduced mABC1 mRNA and protein levels and was associated with significantly attenuated loss of tetramethylrhodamine ethyl ester fluorescence under basal conditions and an increase in trypan blue stained cells. In contrast, adenovirally mediated expression of mABC1 resulted in protection against oxidant stress loss of mitochondrial membrane potential. These results support the notion that mABC1 protein plays a major role in cellular protection against oxidant stress, identifying mABC1 as a novel target for cardioprotective therapeutics.
Figures
References
-
- Klein I, Sarkadi B, Varadi A. An inventory of the human ABC proteins. Biochim Biophys Acta. 1999;1461:237–262. - PubMed
-
- Higgins CF, Linton KJ. ABC transporter: an introduction and overview. In: Holland IB, Cole SPG, Kuchler K, Higgins CF, editors. ABC Proteins. San Diego, Calif: Academic Press; 2003. pp. ix–xvii.
-
- Hogue DL, Liu L, Ling V. Identification and characterization of a mammalian mitochondrial ATP-binding cassette membrane protein. J Mol Biol. 1999;285:379–389. - PubMed
-
- Chloupkova M, LeBard LS, Koeller DM. MDL1 is a high copy suppressor of ATM1: evidence for a role in resistance to oxidative stress. J Mol Biol. 2003;331:155–165. - PubMed
-
- Young L, Leonhard K, Tatsuta T, Trowsdale J, Langer T. Role of the ABC transporter Mdl1 in peptide export from mitochondria. Science. 2001;291:2135–2138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

