Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9
- PMID: 16166626
- PMCID: PMC1265753
- DOI: 10.1128/MCB.25.19.8430-8443.2005
Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9
Abstract
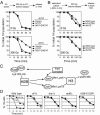
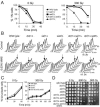
We screened radiation-sensitive yeast mutants for DNA damage checkpoint defects and identified Dot1, the conserved histone H3 Lys 79 methyltransferase. DOT1 deletion mutants (dot1Delta) are G1 and intra-S phase checkpoint defective after ionizing radiation but remain competent for G2/M arrest. Mutations that affect Dot1 function such as Rad6-Bre1/Paf1 pathway gene deletions or mutation of H2B Lys 123 or H3 Lys 79 share dot1Delta checkpoint defects. Whereas dot1Delta alone confers minimal DNA damage sensitivity, combining dot1Delta with histone methyltransferase mutations set1Delta and set2Delta markedly enhances lethality. Interestingly, set1Delta and set2Delta mutants remain G1 checkpoint competent, but set1Delta displays a mild S phase checkpoint defect. In human cells, H3 Lys 79 methylation by hDOT1L likely mediates recruitment of the signaling protein 53BP1 via its paired tudor domains to double-strand breaks (DSBs). Consistent with this paradigm, loss of Dot1 prevents activation of the yeast 53BP1 ortholog Rad9 or Chk2 homolog Rad53 and decreases binding of Rad9 to DSBs after DNA damage. Mutation of Rad9 to alter tudor domain binding to methylated Lys 79 phenocopies the dot1Delta checkpoint defect and blocks Rad53 phosphorylation. These results indicate a key role for chromatin and methylation of histone H3 Lys 79 in yeast DNA damage signaling.
Figures






References
-
- Alcasabas, A. A., A. J. Osborn, J. Bachant, F. Hu, P. J. Werler, K. Bousset, K. Furuya, J. F. Diffley, A. M. Carr, and S. J. Elledge. 2001. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell. Biol. 3:958-965. - PubMed
-
- Bassal, S., and A. El-Osta. 2005. DNA damage detection and repair, and the involvement of epigenetic states. Hum. Mutat. 25:101-109. - PubMed
-
- Bennett, C. B., L. K. Lewis, G. Karthikeyan, K. S. Lobachev, Y. H. Jin, J. F. Sterling, J. R. Snipe, and M. A. Resnick. 2001. Genes required for ionizing radiation resistance in yeast. Nat. Genet. 29:426-434. - PubMed
-
- Bird, A. W., D. Y. Yu, M. G. Pray-Grant, Q. Qiu, K. E. Harmon, P. C. Megee, P. A. Grant, M. M. Smith, and M. F. Christman. 2002. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419:411-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
