Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination
- PMID: 16166645
- PMCID: PMC1265743
- DOI: 10.1128/MCB.25.19.8656-8668.2005
Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination
Abstract
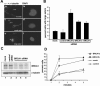
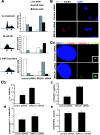
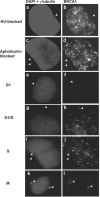
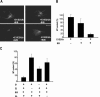
In this study we find that the function of BRCA1 inhibits the microtubule nucleation function of centrosomes. In particular, cells in early S phase have quiescent centrosomes due to BRCA1 activity, which inhibits the association of gamma-tubulin with centrosomes. We find that modification of either of two specific lysine residues (Lys-48 and Lys-344) of gamma-tubulin, a known substrate for BRCA1-dependent ubiquitination activity, led to centrosome hyperactivity. Interestingly, mutation of gamma-tubulin lysine 344 had a minimal effect on centrosome number but a profound effect on microtubule nucleation function, indicating that the processes regulating centrosome duplication and microtubule nucleation are distinct. Using an in vitro aster formation assay, we found that BRCA1-dependent ubiquitination activity directly inhibits microtubule nucleation by centrosomes. Mutant BRCA1 protein that was inactive as a ubiquitin ligase did not inhibit aster formation by the centrosome. Further, a BRCA1 carboxy-terminal truncation mutant that was an active ubiquitin ligase lacked domains critical for the inhibition of centrosome function. These experiments reveal an important new functional assay regulated by the BRCA1-dependent ubiquitin ligase, and the results suggest that the loss of this BRCA1 activity could cause the centrosome hypertrophy and subsequent aneuploidy typically found in breast cancers.
Figures


Similar articles
-
Aurora-A kinase regulates breast cancer associated gene 1 inhibition of centrosome-dependent microtubule nucleation.Cancer Res. 2007 Dec 1;67(23):11186-94. doi: 10.1158/0008-5472.CAN-07-2578. Cancer Res. 2007. PMID: 18056443
-
Identification of domains of BRCA1 critical for the ubiquitin-dependent inhibition of centrosome function.Cancer Res. 2006 Apr 15;66(8):4100-7. doi: 10.1158/0008-5472.CAN-05-4430. Cancer Res. 2006. PMID: 16618730
-
BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number.Mol Cell Biol. 2004 Oct;24(19):8457-66. doi: 10.1128/MCB.24.19.8457-8466.2004. Mol Cell Biol. 2004. PMID: 15367667 Free PMC article.
-
The BRCA1-dependent ubiquitin ligase, gamma-tubulin, and centrosomes.Environ Mol Mutagen. 2009 Oct;50(8):649-53. doi: 10.1002/em.20475. Environ Mol Mutagen. 2009. PMID: 19274767 Review.
-
Regulation of centrosomes by the BRCA1-dependent ubiquitin ligase.Cancer Biol Ther. 2008 Oct;7(10):1540-3. doi: 10.4161/cbt.7.10.7053. Epub 2008 Oct 19. Cancer Biol Ther. 2008. PMID: 18927495 Free PMC article. Review.
Cited by
-
Centrosomes in the DNA damage response--the hub outside the centre.Chromosome Res. 2016 Jan;24(1):35-51. doi: 10.1007/s10577-015-9503-7. Chromosome Res. 2016. PMID: 26614090 Review.
-
BRCA1 regulates microtubule dynamics and taxane-induced apoptotic cell signaling.Oncogene. 2014 Mar 13;33(11):1418-28. doi: 10.1038/onc.2013.85. Epub 2013 Mar 25. Oncogene. 2014. PMID: 23524581 Free PMC article.
-
γ-Tubulin in microtubule nucleation and beyond.Front Cell Dev Biol. 2022 Sep 1;10:880761. doi: 10.3389/fcell.2022.880761. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158181 Free PMC article. Review.
-
The TOG protein Stu2 is regulated by acetylation.PLoS Genet. 2022 Sep 9;18(9):e1010358. doi: 10.1371/journal.pgen.1010358. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36084134 Free PMC article.
-
Mitotic functions for SNAP45, a subunit of the small nuclear RNA-activating protein complex SNAPc.J Biol Chem. 2008 May 23;283(21):14845-56. doi: 10.1074/jbc.M800833200. Epub 2008 Mar 20. J Biol Chem. 2008. PMID: 18356157 Free PMC article.
References
-
- Alberg, A. J., and K. J. Helzlsouer. 1997. Epidemiology, prevention, and early detection of breast cancer. Curr. Opin. Oncol. 9:505-511. - PubMed
-
- Aldaz, H., L. M. Rice, T. Stearns, and D. A. Agard. 2005. Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature 435:523-527. - PubMed
-
- Andersen, J. S., C. J. Wilkinson, T. Mayor, P. Mortensen, E. A. Nigg, and M. Mann. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426:570-574. - PubMed
-
- Anderson, S. F., B. P. Schlegel, T. Nakajima, E. S. Wolpin, and J. D. Parvin. 1998. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat. Genet. 19:254-256. - PubMed
-
- Bornens, M., M. Paintrand, J. Berges, M. C. Marty, and E. Karsenti. 1987. Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskel. 8:238-249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
