pH-Responsive, posttranslational regulation of the Trk1 potassium transporter by the type 1-related Ppz1 phosphatase
- PMID: 16166647
- PMCID: PMC1265754
- DOI: 10.1128/MCB.25.19.8683-8692.2005
pH-Responsive, posttranslational regulation of the Trk1 potassium transporter by the type 1-related Ppz1 phosphatase
Abstract
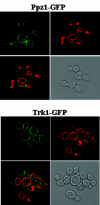
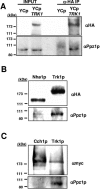
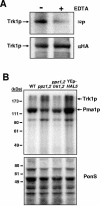
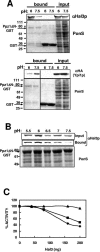
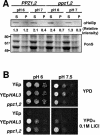
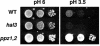
Intracellular pH and K+ concentrations must be tightly controlled because they affect many cellular activities, including cell growth and death. The mechanisms of homeostasis of H+ and K+ are only partially understood. In the yeast Saccharomyces cerevisiae, proton efflux is mediated by the Pma1 H+-ATPase. As this pump is electrogenic, the activity of the Trk1 and -2 K+ uptake system is crucial for sustained Pma1p operation. The coordinated activities of these two systems determine cell volume, turgor, membrane potential, and pH. Genetic evidence indicates that Trk1p is activated by the Hal4 and -5 kinases and inhibited by the Ppz1 and -2 phosphatases, which, in turn, are inhibited by their regulatory subunit, Hal3p. We show that Trk1p, present in plasma membrane "rafts", physically interacts with Ppz1p, that Trk1p is phosphorylated in vivo, and that its level of phosphorylation increases in ppz1 and -2 mutants. Interestingly, both the interaction with and inhibition of Ppz1p by Hal3p are pH dependent. These results are consistent with a model in which the Ppz1-Hal3 interaction is a sensor of intracellular pH that modulates H+ and K+ homeostasis through the regulation of Trk1p activity.
Figures

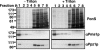

References
-
- Antz, C., T. Bauer, H. Kalbacher, R. Frank, M. Covarrubias, H. R. Kalbitzer, J. P. Ruppersberg, T. Baukrowitz, and B. Fakler. 1999. Control of K+ channel gating by protein phosphorylation: structural switches of the inactivation gate. Nat. Struct. Biol. 6:146-150. - PubMed
-
- Benito, B., F. Portillo, and R. Lagunas. 1992. In vivo activation of the yeast plasma membrane ATPase during nitrogen starvation. Identification of the regulatory domain that controls activation. FEBS Lett. 300:271-274. - PubMed
-
- Bianchini, L., G. L'Allemain, and J. Pouyssegur. 1997. The p42/p44 mitogen-activated protein kinase cascade is determinant in mediating activation of the Na+/H+ exchanger (NHE1 isoform) in response to growth factors. J. Biol. Chem. 272:271-279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
