An interstitial deletion-insertion involving chromosomes 2p25.3 and Xq27.1, near SOX3, causes X-linked recessive hypoparathyroidism
- PMID: 16167084
- PMCID: PMC1201662
- DOI: 10.1172/JCI24156
An interstitial deletion-insertion involving chromosomes 2p25.3 and Xq27.1, near SOX3, causes X-linked recessive hypoparathyroidism
Abstract
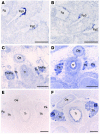
X-linked recessive hypoparathyroidism, due to parathyroid agenesis, has been mapped to a 906-kb region on Xq27 that contains 3 genes (ATP11C, U7snRNA, and SOX3), and analyses have not revealed mutations. We therefore characterized this region by combined analysis of single nucleotide polymorphisms and sequence-tagged sites. This identified a 23- to 25-kb deletion, which did not contain genes. However, DNA fiber-FISH and pulsed-field gel electrophoresis revealed an approximately 340-kb insertion that replaced the deleted fragment. Use of flow-sorted X chromosome-specific libraries and DNA sequence analyses revealed that the telomeric and centromeric breakpoints on X were, respectively, approximately 67 kb downstream of SOX3 and within a repetitive sequence. Use of a monochromosomal somatic cell hybrid panel and metaphase-FISH mapping demonstrated that the insertion originated from 2p25 and contained a segment of the SNTG2 gene that lacked an open reading frame. However, the deletion-insertion [del(X)(q27.1) inv ins (X;2)(q27.1;p25.3)], which represents a novel abnormality causing hypoparathyroidism, could result in a position effect on SOX3 expression. Indeed, SOX3 expression was demonstrated, by in situ hybridization, in the developing parathyroid tissue of mouse embryos between 10.5 and 15.5 days post coitum. Thus, our results indicate a likely new role for SOX3 in the embryonic development of the parathyroid glands.
Figures




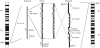
References
-
- Thakker RV. Genetics of endocrine and metabolic disorders: parathyroid. Rev. Endocr. Metab. Disord. 2004;5:37–51. - PubMed
-
- Baldini A. DiGeorge’s syndrome: a gene at last. Lancet. 2003;362:1342–1343. - PubMed
-
- Parkinson DB, Thakker RV. A donor splice site mutation in the parathyroid hormone gene is associated with autosomal recessive hypoparathyroidism. Nat. Genet. 1992;1:149–152. - PubMed
-
- Whyte MP, Weldon VV. Idiopathic hypoparathyroidism presenting with seizures during infancy: X-linked recessive inheritance in a large Missouri kindred. J. Pediatr. 1981;99:608–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

