Mitochondrial ceramide and the induction of apoptosis
- PMID: 16167171
- PMCID: PMC2246044
- DOI: 10.1007/s10863-005-6567-7
Mitochondrial ceramide and the induction of apoptosis
Abstract
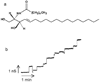
In most cell types, a key event in apoptosis is the release of proapoptotic intermembrane space proteins from mitochondria to the cytoplasm. In general, it is the release of these intermembrane space proteins that is responsible for the activation of caspases and DNases that are responsible for the execution of apoptosis. The mechanism for the increased permeability of the mitochondrial outer membrane during the induction phase of apoptosis is currently unknown and highly debated. This review will focus on one such proposed mechanism, namely, the formation of ceramide channels in the mitochondrial outer membrane. Ceramides are known to play a major regulatory role in apoptosis by inducing the release of proapoptotic proteins from the mitochondria. As mitochondria are known to contain the enzymes responsible for the synthesis and hydrolysis of ceramide, there exists a mechanism for regulating the level of ceramide in mitochondria. In addition, mitochondrial ceramide levels have been shown to be elevated prior to the induction phase of apoptosis. Ceramide has been shown to form large protein permeable channels in planar phospholipid and mitochondrial outer membranes. Thus, ceramide channels are good candidates for the pathway with which proapoptotic proteins are released from mitochondria during the induction phase of apoptosis.
Figures


References
-
- Allouche M, Bettaieb A, Vindis C, Rousse A, Grignon C, Laurent G. Oncogene. 1997;14:1837–1845. - PubMed
-
- Alphonse G, Aloy MT, Broquet P, Gerard JP, Louisot P, Rousson R, Rodriguez-Lafrasse C. Int. J. Radiat. Biol. 2002;78:821–835. - PubMed
-
- Alphonse G, Bionda C, Aloy M-T, Ardail D, Rousson R, Rodriguez-Lafrasse C. Oncogene. 2004;23:2703–2715. - PubMed
-
- Amarante-Mendes GP, Naekyung Kim C, Liu L, Huang Y, Perkins CL, Green DR, Bhalla K. Blood. 1998;91:1700–1705. - PubMed
-
- Ardail D, Popa I, Alcantara K, Pons A, Zanetta JP, Louisot P, Thomas L, Portoukalian J. FEBS Lett. 2001;488:160–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

