Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos
- PMID: 16169962
- PMCID: PMC1255989
- DOI: 10.1104/pp.105.065607
Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos
Abstract
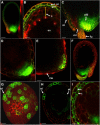
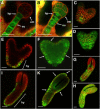
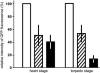
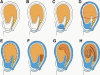
Developing Arabidopsis (Arabidopsis thaliana) seeds and embryos represent a complex set of cell layers and tissues that mediate the transport and partitioning of carbohydrates, amino acids, hormones, and signaling molecules from the terminal end of the funicular phloem to and between these seed tissues and eventually to the growing embryo. This article provides a detailed analysis of the symplastic domains and the cell-to-cell connectivity from the end of the funiculus to the embryo, and within the embryo during its maturation. The cell-to-cell movement of the green fluorescent protein or of mobile and nonmobile green fluorescent protein fusions was monitored in seeds and embryos of plants expressing the corresponding cDNAs under the control of various promoters (SUC2, SUC3, TT12, and GL2) shown to be active in defined seed or embryo cell layers (SUC3, TT12, and GL2) or only outside the developing Arabidopsis seed (AtSUC2). Cell-to-cell movement was also analyzed with the low-molecular-weight fluorescent dye 8-hydroxypyrene-1,3,6-trisulfonate. The analyses presented identify a phloem-unloading domain at the end of the funicular phloem, characterize the entire outer integument as a symplastic extension of the phloem, and describe the inner integument and the globular stage embryo plus the suspensor as symplastic domains. The results also show that, at the time of hypophysis specification, the symplastic connectivity between suspensor and embryo is reduced or interrupted and that the embryo develops from a single symplast (globular and heart stage) to a mature embryo with new symplastic domains.
Figures


References
-
- Becker D, Kemper E, Schell J, Mastersen R (1992) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol 20: 1195–1197 - PubMed
-
- Berleth T, Jürgens G (1993) The role of the MONOPTEROS gene in organizing the basal body region of the Arabidopsis embryo. Development 118: 575–587
-
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 - PubMed
-
- Bowman JL (1993) Embryogenesis. In JL Bowman, ed, Arabidopsis: An Atlas of Morphology and Development. Springer-Verlag, New York, pp 351–401
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

