Arabidopsis RAD51C gene is important for homologous recombination in meiosis and mitosis
- PMID: 16169964
- PMCID: PMC1256004
- DOI: 10.1104/pp.105.065243
Arabidopsis RAD51C gene is important for homologous recombination in meiosis and mitosis
Abstract
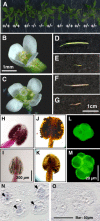
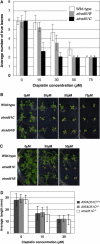
Rad51 is a homolog of the bacterial RecA recombinase, and a key factor in homologous recombination in eukaryotes. Rad51 paralogs have been identified from yeast to vertebrates. Rad51 paralogs are thought to play an important role in the assembly or stabilization of Rad51 that promotes homologous pairing and strand exchange reactions. We previously characterized two RAD51 paralogous genes in Arabidopsis (Arabidopsis thaliana) named AtRAD51C and AtXRCC3, which are homologs of human RAD51C and XRCC3, respectively, and described the interaction of their products in a yeast two-hybrid system. Recent studies showed the involvement of AtXrcc3 in DNA repair and functional role in meiosis. To determine the role of RAD51C in meiotic and mitotic recombination in higher plants, we characterized a T-DNA insertion mutant of AtRAD51C. Although the atrad51C mutant grew normally during vegetative developmental stage, the mutant produced aborted siliques, and their anthers did not contain mature pollen grains. Crossing of the mutant with wild-type plants showed defective male and female gametogeneses as evidenced by lack of seed production. Furthermore, meiosis was severely disturbed in the mutant. The atrad51C mutant also showed increased sensitivity to gamma-irradiation and cisplatin, which are known to induce double-strand DNA breaks. The efficiency of homologous recombination in somatic cells in the mutant was markedly reduced relative to that in wild-type plants.
Figures







Similar articles
-
Differing requirements for the Arabidopsis Rad51 paralogs in meiosis and DNA repair.Plant J. 2005 Feb;41(4):533-45. doi: 10.1111/j.1365-313X.2004.02318.x. Plant J. 2005. PMID: 15686518
-
Arabidopsis RAD51, RAD51C and XRCC3 proteins form a complex and facilitate RAD51 localization on chromosomes for meiotic recombination.PLoS Genet. 2017 May 31;13(5):e1006827. doi: 10.1371/journal.pgen.1006827. eCollection 2017 May. PLoS Genet. 2017. PMID: 28562599 Free PMC article.
-
Arabidopsis Rad51B is important for double-strand DNA breaks repair in somatic cells.Plant Mol Biol. 2005 Apr;57(6):819-33. doi: 10.1007/s11103-005-2187-1. Plant Mol Biol. 2005. PMID: 15952068
-
Double-stranded DNA breaks and gene functions in recombination and meiosis.Cell Res. 2006 May;16(5):402-12. doi: 10.1038/sj.cr.7310052. Cell Res. 2006. PMID: 16699536 Review.
-
The cytogenetics of homologous chromosome pairing in meiosis in plants.Cytogenet Genome Res. 2008;120(3-4):313-9. doi: 10.1159/000121080. Epub 2008 May 23. Cytogenet Genome Res. 2008. PMID: 18504360 Review.
Cited by
-
Genomic analyses of diverse wild and cultivated accessions provide insights into the evolutionary history of jujube.Plant Biotechnol J. 2021 Mar;19(3):517-531. doi: 10.1111/pbi.13480. Epub 2020 Sep 30. Plant Biotechnol J. 2021. PMID: 32946650 Free PMC article.
-
OsRAD51 Plays a Vital Role in Promoting Homologous Recombination in Rice Meiosis.Int J Mol Sci. 2022 Aug 31;23(17):9906. doi: 10.3390/ijms23179906. Int J Mol Sci. 2022. PMID: 36077304 Free PMC article.
-
Structural Aspects of DNA Repair and Recombination in Crop Improvement.Front Genet. 2020 Sep 11;11:574549. doi: 10.3389/fgene.2020.574549. eCollection 2020. Front Genet. 2020. PMID: 33024442 Free PMC article. Review.
-
A 2.09 Mb fragment translocation on chromosome 6 causes abnormalities during meiosis and leads to less seed watermelon.Hortic Res. 2021 Dec 1;8(1):256. doi: 10.1038/s41438-021-00687-9. Hortic Res. 2021. PMID: 34848689 Free PMC article.
-
The retinoblastoma homolog RBR1 mediates localization of the repair protein RAD51 to DNA lesions in Arabidopsis.EMBO J. 2017 May 2;36(9):1279-1297. doi: 10.15252/embj.201694571. Epub 2017 Mar 20. EMBO J. 2017. PMID: 28320735 Free PMC article.
References
-
- Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Armstrong SJ, Christopher F, Franklin H, Jones GH (2001) Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana. J Cell Sci 114: 4207–4217 - PubMed
-
- Baumann P, West SC (1998) Role of human Rad51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci 23: 247–251 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

