Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells
- PMID: 16170032
- PMCID: PMC1343495
- DOI: 10.1158/1535-7163.MCT-05-0082
Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells
Abstract
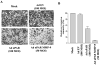
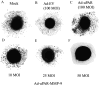
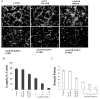
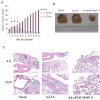
Lung cancer is currently the leading cause of cancer deaths in the United States. Conventional therapeutic treatments, including surgery, chemotherapy, and radiation therapy, have achieved only limited success. The overexpression of proteases, such as urokinase-type plasminogen activator (uPA), its receptor (uPAR), and matrix metalloproteinases (MMP), is correlated with the progression of lung cancer. In the present study, we used a replication-deficient adenovirus capable of expressing antisense uPAR and antisense MMP-9 transcripts to simultaneously down-regulate uPAR and MMP-9 in H1299 cells. Ad-uPAR-MMP-9 infection of H1299 cells resulted in a dose- and time-dependent decrease of uPAR protein levels and MMP-9 activity as determined by Western blotting and gelatin zymography, respectively. Corresponding immunohistochemical analysis also showed that Ad-uPAR-MMP-9 infection inhibited uPAR and MMP-9 expression. As shown by Boyden chamber assay, Ad-uPAR-MMP-9 infection significantly decreased the invasive capacity of H1299 cells compared with mock and Ad-CMV (empty vector)-infected cells in vitro. Furthermore, Ad-uPAR-MMP-9 infection inhibited capillary-like structure formation in H1299 cells cocultured with endothelial cells in a dose-dependent manner compared with mock- and Ad-CMV-infected cells. Ad-uPAR-MMP-9 injection caused the regression of s.c. induced tumors after s.c. injection with H1299 lung cancer cells and inhibited lung metastasis in the metastatic model with A549 cells. These data suggest that Ad-uPAR-MMP-9 shows its antitumor activity against both established and early phases of lung cancer metastases by causing the destruction of the tumor vasculature. In summary, adenovirus-mediated inhibition of uPA-uPAR interaction and MMP-9 on the cell surface may be a promising anti-invasion and antimetastatic strategy for cancer gene therapy.
Figures

References
-
- Shottenfeld D. Epidemiology of lung cancer. In: Pass HI, Mitchell JB, Johnson DH, Turrisi AT, Minna JD, editors. Lung Cancer. Philadelphia: Lippincott Williams & WIlkins; 2003. p.367–88.
-
- Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s–5059s. - PubMed
-
- MacKay AR, Corbitt RH, Hartzler JL, Thorgeirsson UP. Basement membrane type IV collagen degradation: evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res. 1990;50:5997–6001. - PubMed
-
- Dano K, Behrendt N, Brunner N, Ellis V, Ploug M, Pyke C. The urokinse receptor. Protein structure and role in plasminogen activation and cancer invasion. Fibrinolysis. 1994;8:189–203.
-
- Vassalli JD. The urokinase receptor. Fibrinolysis. 1994;8:172–181.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

