The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function
- PMID: 16170200
- PMCID: PMC4667781
- DOI: 10.1074/jbc.M508744200
The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function
Abstract
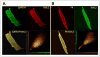
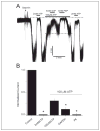
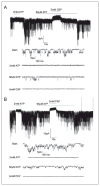
The regulation of ATP-sensitive potassium (K(ATP)) channel activity is complex and a multitude of factors determine their open probability. Physiologically and pathophysiologically, the most important of these are intracellular nucleotides, with a long-recognized role for glycolytically derived ATP in regulating channel activity. To identify novel regulatory subunits of the K(ATP) channel complex, we performed a two-hybrid protein-protein interaction screen, using as bait the mouse Kir6.2 C terminus. Screening a rat heart cDNA library, we identified two potential interacting proteins to be the glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and triose-phosphate isomerase. The veracity of interaction was verified by co-immunoprecipitation techniques in transfected mammalian cells. We additionally demonstrated that pyruvate kinase also interacts with Kir6.2 subunits. The physiological relevance of these interactions is illustrated by the demonstration that native Kir6.2 protein similarly interact with GAPDH and pyruvate kinase in rat heart membrane fractions and that Kir6.2 protein co-localize with these glycolytic enzymes in rat ventricular myocytes. The functional relevance of our findings is demonstrated by the ability of GAPDH or pyruvate kinase substrates to directly block the K(ATP) channel under patch clamp recording conditions. Taken together, our data provide direct evidence for the concept that key enzymes involved in glycolytic ATP production are part of a multisubunit K(ATP) channel protein complex. Our data are consistent with the concept that the activity of these enzymes (possibly by ATP formation in the immediate intracellular microenvironment of this macromolecular K(ATP) channel complex) causes channel closure.
Figures


References
-
- Seino S, Miki T. Prog Biophys Mol Biol. 2003;81:133–176. - PubMed
-
- Inagaki N, Seino S. Jpn J Physiol. 1998;48:397–412. - PubMed
-
- Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR, III, Vandenberg CA. J Biol Chem. 2004;279:22331–22346. - PubMed
-
- Leonoudakis D, Conti LR, Radeke CM, McGuire LM, Vandenberg CA. J Biol Chem. 2004;279:19051–19063. - PubMed
-
- Bers DM. J Mol Cell Cardiol. 2004;37:417–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

