Heat shock proteins as emerging therapeutic targets
- PMID: 16170327
- PMCID: PMC1751210
- DOI: 10.1038/sj.bjp.0706396
Heat shock proteins as emerging therapeutic targets
Abstract
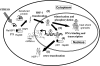
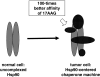
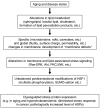
Chaperones (stress proteins) are essential proteins to help the formation and maintenance of the proper conformation of other proteins and to promote cell survival after a large variety of environmental stresses. Therefore, normal chaperone function is a key factor for endogenous stress adaptation of several tissues. However, altered chaperone function has been associated with the development of several diseases; therefore, modulators of chaperone activities became a new and emerging field of drug development. Inhibition of the 90 kDa heat shock protein (Hsp)90 recently emerged as a very promising tool to combat various forms of cancer. On the other hand, the induction of the 70 kDa Hsp70 has been proved to be an efficient help in the recovery from a large number of diseases, such as, for example, ischemic heart disease, diabetes and neurodegeneration. Development of membrane-interacting drugs to modify specific membrane domains, thereby modulating heat shock response, may be of considerable therapeutic benefit as well. In this review, we give an overview of the therapeutic approaches and list some of the key questions of drug development in this novel and promising therapeutic approach.
Figures

References
-
- AGATSUMA T., OGAWA H., AKASAKA K., ASAI A., YAMASHITA Y., MIZUKAMI T., AKINAGA S., SAITOH Y. Halohydrin and oxime derivatives of radicicol: synthesis and antitumor activities. Bioorg. Med. Chem. 2002;10:3445–3454. - PubMed
-
- BAGATELL R., PAINE-MURRIETA G.D., TAYLOR C.W., PULCINI E.J., AKINAGA S., BENJAMIN I.J., WHITESELL L. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin. Cancer Res. 2000;6:3312–3318. - PubMed
-
- BAKER-LEPAIN J.C., REED R.C., NICCHITTA C.V. ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr. Opin. Immunol. 2003;15:89–94. - PubMed
-
- BANERJI U., JUDSON I., WORKMAN P. The clinical applications of heat shock protein inhibitors in cancer – present and future. Curr. Cancer Drug Targets. 2003;3:385–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

