Role of arginine-304 in the diphosphate-triggered active site closure mechanism of trichodiene synthase
- PMID: 16171386
- PMCID: PMC1386727
- DOI: 10.1021/bi0510476
Role of arginine-304 in the diphosphate-triggered active site closure mechanism of trichodiene synthase
Abstract
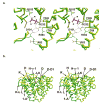
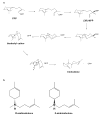
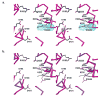
The X-ray crystal structures of R304K trichodiene synthase and its complexes with inorganic pyrophosphate (PP(i)) and aza analogues of the bisabolyl carbocation intermediate are reported. The R304K substitution does not cause large changes in the overall structure in comparison with the wild-type enzyme. The complexes with (R)- and (S)-azabisabolenes and PP(i) bind three Mg2+ ions, and each undergoes a diphosphate-triggered conformational change that caps the active site cavity. This conformational change is only slightly attenuated compared to that of the wild-type enzyme complexed with Mg2+(3)-PP(i), in which R304 donates hydrogen bonds to PP(i) and D101. In R304K trichodiene synthase, K304 does not engage in any hydrogen bond interactions in the unliganded state and it donates a hydrogen bond to only PP(i) in the complex with (R)-azabisabolene; K304 makes no hydrogen bond contacts in its complex with PP(i) and (S)-azabisabolene. Thus, although the R304-D101 hydrogen bond interaction stabilizes diphosphate-triggered active site closure, it is not required for Mg2+(3)-PP(i) binding. Nevertheless, since R304K trichodiene synthase generates aberrant cyclic terpenoids with a 5000-fold reduction in kcat/KM, it is clear that a properly formed R304-D101 hydrogen bond is required in the enzyme-substrate complex to stabilize the proper active site contour, which in turn facilitates cyclization of farnesyl diphosphate for the exclusive formation of trichodiene. Structural analysis of the R304K mutant and comparison with the monoterpene cyclase (+)-bornyl diphosphate synthase suggest that the significant loss in activity results from compromised activation of the PP(i) leaving group.
Figures



Similar articles
-
Exploring biosynthetic diversity with trichodiene synthase.Arch Biochem Biophys. 2007 Oct 15;466(2):260-6. doi: 10.1016/j.abb.2007.06.016. Epub 2007 Jun 28. Arch Biochem Biophys. 2007. PMID: 17678871 Free PMC article.
-
Molecular recognition of the substrate diphosphate group governs product diversity in trichodiene synthase mutants.Biochemistry. 2005 Apr 26;44(16):6153-63. doi: 10.1021/bi050059o. Biochemistry. 2005. PMID: 15835903
-
X-ray crystal structures of D100E trichodiene synthase and its pyrophosphate complex reveal the basis for terpene product diversity.Biochemistry. 2002 Feb 12;41(6):1732-41. doi: 10.1021/bi011960g. Biochemistry. 2002. PMID: 11827517
-
Assembly-Line Catalysis in Bifunctional Terpene Synthases.Acc Chem Res. 2021 Oct 19;54(20):3780-3791. doi: 10.1021/acs.accounts.1c00296. Epub 2021 Jul 13. Acc Chem Res. 2021. PMID: 34254507 Free PMC article. Review.
-
Molecular scaffolds for chemical wizardry: learning nature's rules for terpene cyclases.Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13479-81. doi: 10.1073/pnas.261562898. Proc Natl Acad Sci U S A. 2001. PMID: 11717417 Free PMC article. Review. No abstract available.
Cited by
-
Terpenoid synthase structures: a so far incomplete view of complex catalysis.Nat Prod Rep. 2012 Oct;29(10):1153-75. doi: 10.1039/c2np20059g. Epub 2012 Aug 21. Nat Prod Rep. 2012. PMID: 22907771 Free PMC article. Review.
-
Engineering terpene synthases and their substrates for the biocatalytic production of terpene natural products and analogues.Chem Commun (Camb). 2025 Feb 4;61(12):2468-2483. doi: 10.1039/d4cc05785f. Chem Commun (Camb). 2025. PMID: 39784321 Free PMC article. Review.
-
Exploring biosynthetic diversity with trichodiene synthase.Arch Biochem Biophys. 2007 Oct 15;466(2):260-6. doi: 10.1016/j.abb.2007.06.016. Epub 2007 Jun 28. Arch Biochem Biophys. 2007. PMID: 17678871 Free PMC article.
-
Structural and Chemical Biology of Terpenoid Cyclases.Chem Rev. 2017 Sep 13;117(17):11570-11648. doi: 10.1021/acs.chemrev.7b00287. Epub 2017 Aug 25. Chem Rev. 2017. PMID: 28841019 Free PMC article. Review.
-
Structure-based virtual screening of hypothetical inhibitors of the enzyme longiborneol synthase-a potential target to reduce Fusarium head blight disease.J Mol Model. 2016 Jul;22(7):163. doi: 10.1007/s00894-016-3021-1. Epub 2016 Jun 21. J Mol Model. 2016. PMID: 27324634
References
-
- Wise, M. L., and Croteau, R. In Comprehensive Natural Products Chemistry (Vol. 2) Isoprenoids Including Carotenoids, and Steroids, ed. Cane, D. E., pp 97–153. (Elsevier, Oxford, U. K., 1999).
-
- Cane, D. E. In Comprehensive natural products chemistry (Vol. 2) Isoprenoids including carotenoids, and steroids, ed. Cane, D. E., pp 155–200. (Elsevier, Oxford, U. K., 1999).
-
- MacMillan, J., and Beale, M. H. In Comprehensive natural products chemistry (Vol. 2) Isoprenoids including carotenoids, and steroids, ed. Cane, D. E., pp 217–243. (Elsevier, Oxford, U. K., 1999).
-
- Kawada SZ, Yamashita Y, Ochiai K, Ando K, Iwasaki T, Takaguchi T, Nakano H. Terpentecin and UCT4B, new family of topoisomerase II targeting antitumor antibiotics produced by Streptomyces: producing organism, fermentation and large scale purification. J Antibiot. 1995;48:211–216. - PubMed
-
- Okazaki T, Enokita R, Torikata A, Inukai M, Takeuchi M, Takahashi S, Arai M. Studies on Actinomycetes Producing Pentalenolactone and its New Related Compounds, . Ann Rep Sankyo Res Lab. 1979;31:94–103.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

