Plant virus RNAs. Coordinated recruitment of conserved host functions by (+) ssRNA viruses during early infection events
- PMID: 16172095
- PMCID: PMC1183374
- DOI: 10.1104/pp.105.064105
Plant virus RNAs. Coordinated recruitment of conserved host functions by (+) ssRNA viruses during early infection events
Abstract
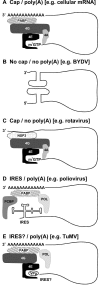
Positive-sense single-stranded RNA viruses have developed strategies to exploit cellular resources at the expense of host mRNAs. The genomes of these viruses display a variety of structures at their 5' and 3' ends that differentiate them from cellular mRNAs. Despite this structural diversity, viral RNAs are still circularized by juxtaposition of their 5' and 3' ends, similar to the process used by cellular mRNAs. Also reminiscent of the mechanisms used by host mRNAs, translation of viral RNAs involves the recruitment of translation initiation factors. However, the roles played by these factors likely differ from those played by cellular mRNAs. In keeping with the general parsimony typical of RNA viruses, these host factors also participate in viral RNA replication. However, the dual use of host factors requires that viral RNA template utilization be regulated to avoid conflict between replication and translation. The molecular composition of the large ribonucleoprotein complexes that form the viral RNA replication and translation machineries likely evolves over the course of infection to allow for switching template use from translation to replication.
Figures
References
-
- Browning KS (2004) Plant translation initiation factors: it is not easy to be green. Biochem Soc Trans 32: 589–591 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

