Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis
- PMID: 16172205
- PMCID: PMC2171544
- DOI: 10.1083/jcb.200503023
Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis
Abstract
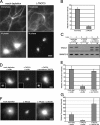
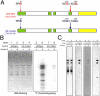
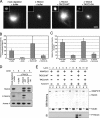
Centrosomes act as sites of microtubule growth, but little is known about how the number and stability of microtubules emanating from a centrosome are controlled during the cell cycle. We studied the role of the TACC3-XMAP215 complex in this process by using purified proteins and Xenopus laevis egg extracts. We show that TACC3 forms a one-to-one complex with and enhances the microtubule-stabilizing activity of XMAP215 in vitro. TACC3 enhances the number of microtubules emanating from mitotic centrosomes, and its targeting to centrosomes is regulated by Aurora A-dependent phosphorylation. We propose that Aurora A regulation of TACC3 activity defines a centrosome-specific mechanism for regulation of microtubule polymerization in mitosis.
Figures




Similar articles
-
Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis.J Cell Biol. 2005 Sep 26;170(7):1057-66. doi: 10.1083/jcb.200504037. Epub 2005 Sep 19. J Cell Biol. 2005. PMID: 16172207 Free PMC article.
-
Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules.J Cell Biol. 2005 Sep 26;170(7):1039-46. doi: 10.1083/jcb.200504097. J Cell Biol. 2005. PMID: 16186253 Free PMC article.
-
Xenopus TACC3/maskin is not required for microtubule stability but is required for anchoring microtubules at the centrosome.Mol Biol Cell. 2008 Aug;19(8):3347-56. doi: 10.1091/mbc.e07-11-1204. Epub 2008 May 28. Mol Biol Cell. 2008. PMID: 18508920 Free PMC article.
-
The role of TACC3 in mitotic spindle organization.Cytoskeleton (Hoboken). 2017 Oct;74(10):369-378. doi: 10.1002/cm.21388. Epub 2017 Aug 23. Cytoskeleton (Hoboken). 2017. PMID: 28745816 Review.
-
Polar expeditions--provisioning the centrosome for mitosis.Nat Cell Biol. 2003 Jun;5(6):505-11. doi: 10.1038/ncb0603-505. Nat Cell Biol. 2003. PMID: 12776127 Review.
Cited by
-
Xenopus TACC1 is a microtubule plus-end tracking protein that can regulate microtubule dynamics during embryonic development.Cytoskeleton (Hoboken). 2015 May;72(5):225-34. doi: 10.1002/cm.21224. Cytoskeleton (Hoboken). 2015. PMID: 26012630 Free PMC article.
-
NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment.Mol Cell Biol. 2007 Jan;27(1):352-67. doi: 10.1128/MCB.00878-06. Epub 2006 Oct 23. Mol Cell Biol. 2007. PMID: 17060449 Free PMC article.
-
TACC3 Regulates Microtubule Plus-End Dynamics and Cargo Transport in Interphase Cells.Cell Rep. 2020 Jan 7;30(1):269-283.e6. doi: 10.1016/j.celrep.2019.12.025. Cell Rep. 2020. PMID: 31914393 Free PMC article.
-
The centrosomal adaptor TACC3 and the microtubule polymerase chTOG interact via defined C-terminal subdomains in an Aurora-A kinase-independent manner.J Biol Chem. 2014 Jan 3;289(1):74-88. doi: 10.1074/jbc.M113.532333. Epub 2013 Nov 22. J Biol Chem. 2014. PMID: 24273164 Free PMC article.
-
Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain.EMBO J. 2011 May 4;30(9):1690-704. doi: 10.1038/emboj.2011.81. Epub 2011 Mar 25. EMBO J. 2011. PMID: 21441895 Free PMC article.
References
-
- Ashford, A.J., S.S. Andersen, and A.A. Hyman. 1998. Preparation of tubulin from bovine brain. Cell Biology: A Laboratory Handbook. Vol. 2. 2nd ed. J.E. Celis, editor. Academic Press/UK, London. 205–212.
-
- Bellanger, J.M., and P. Gönczy. 2003. TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr. Biol. 13:1488–1498. - PubMed
-
- Belmont, L.D., A.A. Hyman, K.E. Sawin, and T.J. Mitchison. 1990. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 62:579–589. - PubMed
-
- Cheeseman, I.M., S. Anderson, M. Jwa, E.M. Green, J. Kang, J.R. Yates, C.S. Chan, D.G. Drubin, and G. Barnes. 2002. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 111:163–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

