Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management
- PMID: 16172252
- PMCID: PMC2715340
- DOI: 10.1165/rcmb.F305
Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management
Figures
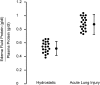

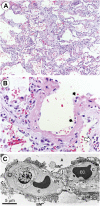
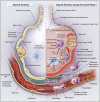


References
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319–323. - PubMed
-
- Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005;171:995–1001. - PubMed
-
- Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet J-F, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002;346:1281–1286. - PubMed
-
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Joffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003;167:1027–1035. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

