Modifier loci condition autoimmunity provoked by Aire deficiency
- PMID: 16172259
- PMCID: PMC2212943
- DOI: 10.1084/jem.20050693
Modifier loci condition autoimmunity provoked by Aire deficiency
Abstract
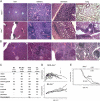
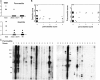
Loss of function mutations in the autoimmune regulator (Aire) gene in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients and mutant mice lead to autoimmune manifestations that segregate as a monogenic trait, but with wide variation in the spectrum of organs targeted. To investigate the cause of this variability, the Aire knockout mutation was backcrossed to mice of diverse genetic backgrounds. The background loci strongly influenced the pattern of organs that were targeted (stomach, eye, pancreas, liver, ovary, thyroid, and salivary gland) and the severity of the targeting (particularly strong on the nonobese diabetic background, but very mild on the C57BL/6 background). Autoantibodies mimicked the disease pattern, with oligoclonal reactivity to a few antigens that varied between Aire-deficient strains. Congenic analysis and a whole genome scan showed that autoimmunity to each organ had a distinctive pattern of genetic control and identified several regions that controlled the pattern of targeting, including the major histocompatibility complex and regions of Chr1 and Chr3 previously identified in controlling type 1 diabetes.
Figures




References
-
- Bach, J.F. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347:911–920. - PubMed
-
- Kishimoto, H., and J. Sprent. 2001. A defect in central tolerance in NOD mice. Nat. Immunol. 2:1025–1031. - PubMed
-
- Liston, A., S. Lesage, D.H. Gray, L.A. O'Reilly, A. Strasser, A.M. Fahrer, R.L. Boyd, J. Wilson, A.G. Baxter, E.M. Gallo, et al. 2004. Generalized resistance to thymic deletion in the NOD mouse; a polygenic trait characterized by defective induction of Bim. Immunity. 21:817–830. - PubMed
-
- Choisy-Rossi, C.M., T.M. Holl, M.A. Pierce, H.D. Chapman, and D.V. Serreze. 2004. Enhanced pathogenicity of diabetogenic T cells escaping a non-MHC gene-controlled near death experience. J. Immunol. 173:3791–3800. - PubMed
-
- Zucchelli, S., P. Holler, T. Yamagata, M. Roy, C. Benoist, and D. Mathis. 2005. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity. 22:385–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

