Rapid hydrolysis of ATP by mitochondrial F1-ATPase correlates with the filling of the second of three catalytic sites
- PMID: 16172372
- PMCID: PMC1236596
- DOI: 10.1073/pnas.0507139102
Rapid hydrolysis of ATP by mitochondrial F1-ATPase correlates with the filling of the second of three catalytic sites
Abstract
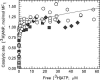
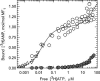
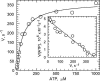
Strong positive catalytic cooperativity is a central feature of the binding change mechanism for F1-ATPases. However, a detail of the mechanism that remains controversial is whether the kinetic enhancement derived from using substrate-binding energy at one catalytic site to promote product release from another site occurs upon the filling of the second or third of three catalytic sites on F1. To address this question, we compare the ATP concentration dependence of the rate of ATP hydrolysis by F1 from beef heart mitochondria to the ATP concentration dependence of the level of occupancy of catalytic sites during steady-state catalysis as measured by a centrifuge filtration assay. A single Km(ATP) is observed at 77 +/- 6 microM. Analysis of the nucleotide-binding data shows that half-maximal occupancy of a second catalytic site occurs at 78 +/- 18 microM ATP. We conclude that ATP binding to a second catalytic site is sufficient to support rapid rates of catalysis.
Figures

Similar articles
-
Bi-site activation occurs with the native and nucleotide-depleted mitochondrial F1-ATPase.Biochem J. 1998 Mar 1;330 ( Pt 2)(Pt 2):1037-43. doi: 10.1042/bj3301037. Biochem J. 1998. PMID: 9480927 Free PMC article.
-
Kinetic mechanism of Fo x F1 mitochondrial ATPase: Mg2+ requirement for Mg x ATP hydrolysis.Biochemistry (Mosc). 1999 Oct;64(10):1128-37. Biochemistry (Mosc). 1999. PMID: 10561559
-
Evidence for catalytic cooperativity during ATP hydrolysis by beef heart F1-ATPase. Kinetics and binding studies with the photoaffinity label BzATP.J Biol Chem. 1987 Oct 5;262(28):13765-72. J Biol Chem. 1987. PMID: 2888764
-
Toward an adequate scheme for the ATP synthase catalysis.Biochemistry (Mosc). 2001 Oct;66(10):1058-66. doi: 10.1023/a:1012420610963. Biochemistry (Mosc). 2001. PMID: 11736627 Review.
-
Mechanism of ATP synthesis by mitochondrial ATP synthase from beef heart.J Bioenerg Biomembr. 1994 Dec;26(6):627-30. doi: 10.1007/BF00831537. J Bioenerg Biomembr. 1994. PMID: 7721724 Review.
Cited by
-
Ecto-F₁-ATPase: a moonlighting protein complex and an unexpected apoA-I receptor.World J Gastroenterol. 2010 Dec 21;16(47):5925-35. doi: 10.3748/wjg.v16.i47.5925. World J Gastroenterol. 2010. PMID: 21157968 Free PMC article. Review.
-
Studies of nucleotide binding to the catalytic sites of Escherichia coli betaY331W-F1-ATPase using fluorescence quenching.Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4327-31. doi: 10.1073/pnas.0700078104. Epub 2007 Mar 5. Proc Natl Acad Sci U S A. 2007. PMID: 17360523 Free PMC article.
-
Engineering a light-controlled F1 ATPase using structure-based protein design.PeerJ. 2016 Jul 28;4:e2286. doi: 10.7717/peerj.2286. eCollection 2016. PeerJ. 2016. PMID: 27547581 Free PMC article.
-
Asymmetric structure of the yeast F1 ATPase in the absence of bound nucleotides.J Biol Chem. 2009 Apr 17;284(16):10546-51. doi: 10.1074/jbc.M900544200. Epub 2009 Feb 20. J Biol Chem. 2009. PMID: 19233840 Free PMC article.
-
ATP hydrolysis-driven H(+) translocation is stimulated by sulfate, a strong inhibitor of mitochondrial ATP synthesis.J Bioenerg Biomembr. 2008 Aug;40(4):269-79. doi: 10.1007/s10863-008-9177-3. Epub 2008 Oct 10. J Bioenerg Biomembr. 2008. PMID: 18846414
References
-
- Abrahams, J. P., Leslie, A. G., Lutter, R. & Walker, J. E. (1994) Nature 370, 621–628. - PubMed
-
- Boyer, P. D. (1997) Annu. Rev. Biochem. 66, 717–749. - PubMed
-
- Kayalar, C., Rosing, J. & Boyer, P. D. (1977) J. Biol. Chem. 252, 2486–2491. - PubMed
-
- Boyer, P. D. & Kohlbrenner, W. E. (1981) in Energy Coupling in Photosynthesis, eds. Selman, B. & Selman-Reiner, S. (Elsevier, New York), pp. 231–240.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

