Pathogenic myelin oligodendrocyte glycoprotein antibodies recognize glycosylated epitopes and perturb oligodendrocyte physiology
- PMID: 16172404
- PMCID: PMC1236555
- DOI: 10.1073/pnas.0504979102
Pathogenic myelin oligodendrocyte glycoprotein antibodies recognize glycosylated epitopes and perturb oligodendrocyte physiology
Abstract
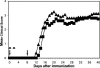
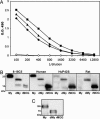
Antibodies to myelin components are routinely detected in multiple sclerosis patients. However, their presence in some control subjects has made it difficult to determine their contribution to disease pathogenesis. Immunization of C57BL/6 mice with either rat or human myelin oligodendrocyte glycoprotein (MOG) leads to experimental autoimmune encephalomyelitis (EAE) and comparable titers of anti-MOG antibodies as detected by ELISA. However, only immunization with human (but not rat) MOG results in a B cell-dependent EAE. In this study, we demonstrate that these pathogenic and nonpathogenic anti-MOG antibodies have a consistent array of differences in their recognition of antigenic determinants and biological effects. Specifically, substituting proline at position 42 with serine in human MOG (as in rat MOG) eliminates the B cell requirement for EAE. All MOG proteins analyzed induced high titers of anti-MOG (tested by ELISA), but only antisera from mice immunized with unmodified human MOG were encephalitogenic in primed B cell-deficient mice. Nonpathogenic IgGs bound recombinant mouse MOG and deglycosylated MOG in myelin (tested by Western blot), but only pathogenic IgGs bound glycosylated MOG. Only purified IgG to human MOG bound to live rodent oligodendrocytes in culture and, after cross-linking, induced repartitioning of MOG into lipid rafts, followed by dramatic changes in cell morphology. The data provide a strong link between in vivo and in vitro observations regarding demyelinating disease, further indicate a biochemical mechanism for anti-MOG-induced demyelination, and suggest in vitro tools for determining autoimmune antibody pathogenicity in multiple sclerosis patients.
Figures


References
-
- Haase, C. G., Guggenmos, J., Brehm, U., Andersson, M., Olsson, T., Reindl, M., Schneidewind, J. M., Zettl, U. K., Heidenreich, F., Berger, T., et al. (2001) J. Neuroimmunol. 114, 220-225. - PubMed
-
- Lindert, R. B., Haase, C. G., Brehm, U., Linington, C., Wekerle, H. & Hohlfeld, R. (1999) Brain 122, 2089-2100. - PubMed
-
- Genain, C. P., Cannella, B., Hauser, S. L. & Raine, C. S. (1999) Nat. Med. 5, 170-175. - PubMed
-
- Lassmann, H. (2004) in Myelin Biology and Disorders, ed. Lazzarini, R. A. (Elsevier Academic, San Diego), Vol. 2, pp. 733-762.
-
- Lucchinetti, C., Bruck, W., Parisi, J., Scheithauer, B., Rodriguez, M. & Lassmann, H. (2000) Ann. Neurol. 47, 707-717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

