Gene expression profiling reveals a signaling role of glutathione in redox regulation
- PMID: 16172407
- PMCID: PMC1236550
- DOI: 10.1073/pnas.0504398102
Gene expression profiling reveals a signaling role of glutathione in redox regulation
Abstract
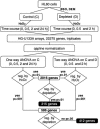
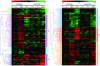
Proteins can form reversible mixed disulfides with glutathione (GSH). It has been hypothesized that protein glutathionylation may represent a mechanism of redox regulation, in a fashion similar to that mediated by protein phosphorylation. We investigated whether GSH has a signaling role in the response of HL60 cells to hydrogen peroxide (H2O2), in addition to its obvious antioxidant role. We identified early changes in gene expression induced at different times by H2O2 treatment, under conditions that increase protein glutathionylation and minimal toxicity. We then investigated the effect of prior GSH depletion by buthionine sulfoximine and diethylmaleate on this response. The analysis revealed 2,016 genes regulated by H2O2. Of these, 215 genes showed GSH-dependent expression changes, classifiable into four clusters displaying down- or up-regulation by H2O2, either potentiated or inhibited by GSH depletion. The modulation of 20 selected genes was validated by real-time RT-PCR. The biological process categories overrepresented in the largest cluster (genes whose up-regulation was inhibited by GSH depletion) were NF-kappaB activation, transcription, and DNA methylation. This cluster also included several cytokine and chemokine ligands and receptors, the redox regulator thioredoxin interacting protein, and the histone deacetylase sirtuin. The cluster of genes whose up-regulation was potentiated by GSH depletion included two HSPs (HSP40 and HSP70) and the AP-1 transcription factor components Fos and FosB. This work demonstrates that GSH, in addition to its antioxidant and protective function against oxidative stress, has a specific signaling role in redox regulation.
Figures

References
-
- Meister, A. & Anderson, M. E. (1983) Annu. Rev. Biochem. 52, 711-760. - PubMed
-
- Fratelli, M., Gianazza, E. & Ghezzi, P. (2004) Exp. Rev. Proteom. 1, 89-100. - PubMed
-
- Ghezzi, P. (2005) Free Radical Res. 39, 573-580. - PubMed
-
- Shelton, M. D., Chock, P. B. & Mieyal, J. J. (2005) Antioxid. Redox Signal. 7, 348-366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

