Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells
- PMID: 16173059
- PMCID: PMC2291358
- DOI: 10.1002/jbt.20084
Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells
Abstract
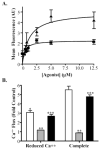
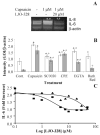
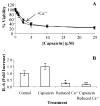
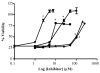
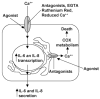
Activation of the capsaicin receptor (VR1 or TRPV1) in bronchial epithelial cells by capsaicinoids and other vanilloids promotes pro-inflammatory cytokine production and cell death. The purpose of this study was to investigate the role of TRPV1-mediated calcium flux from extracellular sources as an initiator of these responses and to define additional cellular pathways that control cell death. TRPV1 antagonists and reduction of calcium concentrations in treatment solutions attenuated calcium flux, induction of interleukin-6 and 8 gene expression, and IL-6 secretion by cells treated with capsaicin or resiniferatoxin. Most TRPV1 antagonists also attenuated cell death, but the relative potency and extent of protection did not directly correlate with inhibition of total calcium flux. Treatment solutions with reduced calcium content or chelators had no effect on cytotoxicity. Inhibitors of arachidonic acid metabolism and cyclo-oxygenases also prevented cell death indicating that TRPV1 agonists disrupted basal arachidonic acid metabolism and altered cyclo-oxygenase function via a TRPV1-dependent mechanism in order to produce toxicity. These data confirm previous results demonstrating calcium flux through TRPV1 acts as a trigger for cytokine production by vanilloids, and provides new mechanistic insights on mechanisms of cell death produced by TRPV1 agonists in respiratory epithelial cells.
Copyright 2005 Wiley Periodicals, Inc
Figures

Similar articles
-
TRPV1 antagonists elevate cell surface populations of receptor protein and exacerbate TRPV1-mediated toxicities in human lung epithelial cells.Toxicol Sci. 2006 Jan;89(1):278-86. doi: 10.1093/toxsci/kfi292. Epub 2005 Aug 24. Toxicol Sci. 2006. PMID: 16120755 Free PMC article.
-
Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors.Toxicol Sci. 2003 May;73(1):170-81. doi: 10.1093/toxsci/kfg044. Toxicol Sci. 2003. PMID: 12721390 Free PMC article.
-
TRPV1 mediates capsaicin-stimulated metabolic activity but not cell death or inhibition of interleukin-1β release in human THP-1 monocytes.Toxicol Appl Pharmacol. 2018 Dec 1;360:9-17. doi: 10.1016/j.taap.2018.09.025. Epub 2018 Sep 20. Toxicol Appl Pharmacol. 2018. PMID: 30244119
-
Structure-activity relationship of capsaicin analogs and transient receptor potential vanilloid 1-mediated human lung epithelial cell toxicity.J Pharmacol Exp Ther. 2011 May;337(2):400-10. doi: 10.1124/jpet.110.178491. Epub 2011 Feb 22. J Pharmacol Exp Ther. 2011. PMID: 21343315 Free PMC article.
-
Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells.J Pharmacol Exp Ther. 2007 Jun;321(3):830-8. doi: 10.1124/jpet.107.119412. Epub 2007 Mar 1. J Pharmacol Exp Ther. 2007. PMID: 17332266
Cited by
-
Transient receptor potential vanilloid-1 (TRPV1) is a mediator of lung toxicity for coal fly ash particulate material.Mol Pharmacol. 2012 Mar;81(3):411-9. doi: 10.1124/mol.111.076067. Epub 2011 Dec 9. Mol Pharmacol. 2012. PMID: 22155782 Free PMC article.
-
Metabolism of capsaicinoids by P450 enzymes: a review of recent findings on reaction mechanisms, bio-activation, and detoxification processes.Drug Metab Rev. 2006;38(4):685-706. doi: 10.1080/03602530600959557. Drug Metab Rev. 2006. PMID: 17145696 Free PMC article. Review.
-
Characterization of Transient Receptor Potential Vanilloid-1 (TRPV1) Variant Activation by Coal Fly Ash Particles and Associations with Altered Transient Receptor Potential Ankyrin-1 (TRPA1) Expression and Asthma.J Biol Chem. 2016 Nov 25;291(48):24866-24879. doi: 10.1074/jbc.M116.746156. Epub 2016 Oct 7. J Biol Chem. 2016. PMID: 27758864 Free PMC article.
-
TRPV1 antagonists elevate cell surface populations of receptor protein and exacerbate TRPV1-mediated toxicities in human lung epithelial cells.Toxicol Sci. 2006 Jan;89(1):278-86. doi: 10.1093/toxsci/kfi292. Epub 2005 Aug 24. Toxicol Sci. 2006. PMID: 16120755 Free PMC article.
-
Nobilamides A-H, long-acting transient receptor potential vanilloid-1 (TRPV1) antagonists from mollusk-associated bacteria.J Med Chem. 2011 Jun 9;54(11):3746-55. doi: 10.1021/jm101621u. Epub 2011 May 16. J Med Chem. 2011. PMID: 21524089 Free PMC article.
References
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–28619. - PubMed
-
- Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2004;33:479–487. - PubMed
-
- Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, Barton AJ, Medhurst AD, Smith GD, Topp S, Murdock P, Sanger GJ, Terrett J, Jenkins O, Benham CD, Randall AD, Gloger IS, Davis JB. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–215. - PubMed
-
- Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304:217–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

