Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides
- PMID: 16173809
- PMCID: PMC2539111
- DOI: 10.1021/bc050201y
Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides
Abstract
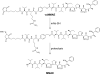
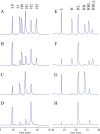
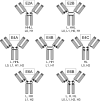
Site-specific conjugation of small molecules and enzymes to monoclonal antibodies has broad utility in the formation of conjugates for therapeutic, diagnostic, or structural applications. Precise control over the location of conjugation would yield highly homogeneous materials that could have improved biological properties. We describe for the first time chemical reduction and oxidation methods that lead to preferential cleavage of particular monoclonal antibody interchain disulfides using the anti-CD30 IgG1 monoclonal antibody cAC10. Alkylation of the resulting cAC10 cysteine thiols with the potent antimitotic agent monomethyl auristatin E (MMAE) enabled the assignment of drug conjugation location by purification with hydrophobic interaction chromatography followed by analysis using reversed-phase HPLC and capillary electrophoresis. These analytical methods demonstrated that treating cAC10 with reducing agents such as DTT caused preferential reduction of heavy-light chain disulfides, while reoxidation of fully reduced cAC10 interchain disulfides caused preferential reformation of heavy-light chain disulfides. Following MMAE conjugation, the resulting conjugates had isomeric homogeneity as high as 60-90%, allowing for control of the distribution of molecular species. The resulting conjugates are highly active both in vitro and in vivo and are well tolerated at efficacious doses.
Figures




References
-
- Senter PD, Springer CJ. Selective activation of anticancer prodrugs by monoclonal antibody-enzyme conjugates. Adv Drug Deliv Rev. 2001;53:247–64. - PubMed
-
- Sharkey RM, Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46(Suppl 1):115S–27S. - PubMed
-
- Bohdiewicz PJ. Indium-111 satumomab pendetide: the first FDA-approved monoclonal antibody for tumor imaging. J Nucl Med Technol. 1998;26:155–63. quiz 170−1. - PubMed
-
- Van de Wiele C, Revets H, Mertens N. Radioimmunoimaging. Advances and prospects. Q J Nucl Med Mol Imaging. 2004;48:317–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

