Site-specific PEGylation of engineered cysteine analogues of recombinant human granulocyte-macrophage colony-stimulating factor
- PMID: 16173810
- PMCID: PMC1373678
- DOI: 10.1021/bc050172r
Site-specific PEGylation of engineered cysteine analogues of recombinant human granulocyte-macrophage colony-stimulating factor
Abstract
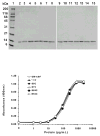
Granulocyte macrophage colony-stimulating factor (GM-CSF) stimulates proliferation of hematopoietic cells of the macrophage and granulocyte lineages and is used clinically to treat neutropenia and other myeloid disorders. Because of its short circulating half-life, GM-CSF is administered to patients by daily injection. We describe here the engineering of highly potent, long-acting human GM-CSF proteins through site-specific modification of GM-CSF cysteine analogues with a cysteine-reactive poly(ethylene glycol) (PEG) reagent. Thirteen cysteine analogues of GM-CSF were constructed, primarily in nonhelical regions of the protein believed to lie away from the major receptor binding sites. The GM-CSF cysteine analogues were properly processed but insoluble following secretion into the Escherichia coli periplasm. The proteins were refolded and purified by column chromatography. Ten of the cysteine analogues could be modified with a 5-kDa maleimide PEG, and seven of the mono-PEGylated proteins were purified by ion-exchange column chromatography. Biological activities of the 13 cysteine analogues and 7 PEGylated cysteine analogues were comparable to that of wild-type GM-CSF in an in vitro cell proliferation assay using human TF-1 cells. One cysteine analogue was modified with larger 10-, 20-, and 40-kDa PEGs, with only minimal loss of in vitro bioactivity. Pharmacokinetic experiments in rats demonstrated that the PEGylated proteins had up to 47-fold longer circulating half-lives than wild-type GM-CSF. These data demonstrate the utility of site-specific PEGylation for creating highly potent, long-acting GM-CSF analogues and provide further evidence that the nonhelical regions of human GM-CSF examined are largely nonessential for biological activity of the protein.
Figures





Similar articles
-
Characterization of a Long-Acting Site-Specific PEGylated Murine GM-CSF Analog and Analysis of Its Hematopoietic Properties in Normal and Cyclophosphamide-Treated Neutropenic Rats.Protein J. 2020 Apr;39(2):160-173. doi: 10.1007/s10930-020-09894-0. Protein J. 2020. PMID: 32172395
-
Preparation and characterization of monopegylated human granulocyte-macrophage colony-stimulating factor.J Interferon Cytokine Res. 2008 Feb;28(2):101-12. doi: 10.1089/jir.2006.0167. J Interferon Cytokine Res. 2008. PMID: 18279105
-
A long-acting, highly potent interferon alpha-2 conjugate created using site-specific PEGylation.Bioconjug Chem. 2005 Jan-Feb;16(1):200-7. doi: 10.1021/bc049713n. Bioconjug Chem. 2005. PMID: 15656592
-
Structure-based design of mimetics for granulocyte-macrophage colony stimulating factor (GM-CSF).Curr Pharm Des. 2002;8(24):2185-99. doi: 10.2174/1381612023393198. Curr Pharm Des. 2002. PMID: 12369862 Review.
-
The biology of granulocyte-macrophage colony-stimulating factor: effects on hematopoietic and nonhematopoietic cells.Dev Biol. 1992 Jun;151(2):352-67. doi: 10.1016/0012-1606(92)90175-g. Dev Biol. 1992. PMID: 1601172 Review.
Cited by
-
Development of Peptide Vaccines in Dengue.Curr Pharm Des. 2018;24(11):1157-1173. doi: 10.2174/1381612823666170913163904. Curr Pharm Des. 2018. PMID: 28914200 Free PMC article. Review.
-
Sortase-catalyzed transformations that improve the properties of cytokines.Proc Natl Acad Sci U S A. 2011 Feb 22;108(8):3169-74. doi: 10.1073/pnas.1016863108. Epub 2011 Feb 4. Proc Natl Acad Sci U S A. 2011. PMID: 21297034 Free PMC article.
-
Allylic selenosulfide rearrangement: a method for chemical ligation to cysteine and other thiols.J Am Chem Soc. 2006 Mar 1;128(8):2544-5. doi: 10.1021/ja057521c. J Am Chem Soc. 2006. PMID: 16492032 Free PMC article.
-
Cytokine refacing effect reduces granulocyte macrophage colony-stimulating factor susceptibility to antibody neutralization.Protein Eng Des Sel. 2015 Oct;28(10):461-6. doi: 10.1093/protein/gzv019. Epub 2015 Apr 7. Protein Eng Des Sel. 2015. PMID: 25855658 Free PMC article.
-
In silico designing of a new cysteine analogue of hirudin variant 3 for site specific PEGylation.Res Pharm Sci. 2017 Feb;12(1):60-66. doi: 10.4103/1735-5362.199048. Res Pharm Sci. 2017. PMID: 28255315 Free PMC article.
References
-
- Cebon JS, Lieschke GJ. Granulocyte-macrophage colony-stimulating factor for cancer treatment. Oncology. 1994;51:177–188. - PubMed
-
- Armitage JO. Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1998;92:4491–4507. - PubMed
-
- Dieckgraefe B.K. and Korzenik J.R. Treatment of active Crohn's disease with recombinant human granulocyte-macrophage colony-stimulating factor. Lancet. 2002;360:1478–1480. - PubMed
-
- Spitler LE, Grossbard ML, Ernstoff MS, Silver G, Jacobs M, Hayes FA, Soong SJ. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18:1603–1605. - PubMed
-
- Abuchowski A, Kazo GM, Verhoest CR, Van Es T, Kafkewitz D, Nucci ML, Viau AT, Davis FF. Cancer therapy with chemically modified enzymes. I Antitumor proterties of polyethylene glycol- asparaginase conjugates. Cancer Biochem Biophys. 1984;7:175–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

