Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity
- PMID: 16174037
- PMCID: PMC4386657
- DOI: 10.1111/j.1525-142X.2005.05048.x
Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity
Abstract
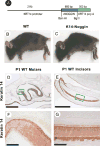
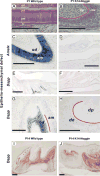
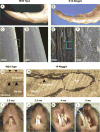
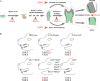
During development and evolution, the morphology of ectodermal organs can be modulated so that an organism can adapt to different environments. We have proposed that morphoregulation can be achieved by simply tilting the balance of molecular activity. We test the principles by analyzing the effects of partial downregulation of Bmp signaling in oral and dental epithelia of the keratin 14-Noggin transgenic mouse. We observed a wide spectrum of tooth phenotypes. The dental formula changed from 1.0.0.3/1.0.0.3 to 1.0.0.2(1)/1.0.0.0. All mandibular and M3 maxillary molars were selectively lost because of the developmental block at the early bud stage. First and second maxillary molars were reduced in size, exhibited altered crown patterns, and failed to form multiple roots. In these mice, incisors were not transformed into molars. Histogenesis and differentiation of ameloblasts and odontoblasts in molars and incisors were abnormal. Lack of enamel caused misocclusion of incisors, leading to deformation and enlargement in size. Therefore, subtle differences in the level, distribution, and timing of signaling molecules can have major morphoregulatory consequences. Modulation of Bmp signaling exemplifies morphoregulation hypothesis: simple alteration of key signaling pathways can be used to transform a prototypical conical-shaped tooth into one with complex morphology. The involvement of related pathways and the implication of morphoregulation in tooth evolution are discussed.
Figures




Similar articles
-
A role for suppressed incisor cuspal morphogenesis in the evolution of mammalian heterodont dentition.Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):92-7. doi: 10.1073/pnas.0907236107. Epub 2009 Dec 14. Proc Natl Acad Sci U S A. 2010. PMID: 20018657 Free PMC article.
-
Tooth morphogenesis and ameloblast differentiation are regulated by micro-RNAs.Dev Biol. 2010 Apr 15;340(2):355-68. doi: 10.1016/j.ydbio.2010.01.019. Epub 2010 Jan 25. Dev Biol. 2010. PMID: 20102707
-
Noggin is required for early development of murine upper incisors.J Dent Res. 2012 Apr;91(4):394-400. doi: 10.1177/0022034511435939. Epub 2012 Feb 2. J Dent Res. 2012. PMID: 22302143 Free PMC article.
-
Affecting tooth morphology and renewal by fine-tuning the signals mediating cell and tissue interactions.Novartis Found Symp. 2007;284:142-53; discussion 153-63. doi: 10.1002/9780470319390.ch10. Novartis Found Symp. 2007. PMID: 17710852 Review.
-
Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation.Adv Dent Res. 2001 Aug;15:14-8. doi: 10.1177/08959374010150010401. Adv Dent Res. 2001. PMID: 12640732 Review.
Cited by
-
BMP activity is required for tooth development from the lamina to bud stage.J Dent Res. 2012 Jul;91(7):690-5. doi: 10.1177/0022034512448660. Epub 2012 May 16. J Dent Res. 2012. PMID: 22592126 Free PMC article.
-
Tissue Engineering Approaches for Enamel, Dentin, and Pulp Regeneration: An Update.Stem Cells Int. 2020 Feb 25;2020:5734539. doi: 10.1155/2020/5734539. eCollection 2020. Stem Cells Int. 2020. PMID: 32184832 Free PMC article. Review.
-
The Pitx2:miR-200c/141:noggin pathway regulates Bmp signaling and ameloblast differentiation.Development. 2013 Aug;140(16):3348-59. doi: 10.1242/dev.089193. Epub 2013 Jul 17. Development. 2013. PMID: 23863486 Free PMC article.
-
Disruption of Smad4 in odontoblasts causes multiple keratocystic odontogenic tumors and tooth malformation in mice.Mol Cell Biol. 2009 Nov;29(21):5941-51. doi: 10.1128/MCB.00706-09. Epub 2009 Aug 24. Mol Cell Biol. 2009. PMID: 19703995 Free PMC article.
-
Tooth Crown Morphology in Turner and Klinefelter Syndrome Individuals from a Croatian Sample.Acta Stomatol Croat. 2019 Jun;53(2):106-118. doi: 10.15644/asc53/2/2. Acta Stomatol Croat. 2019. PMID: 31341318 Free PMC article.
References
-
- Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (bmps) in the developing mouse tooth suggest poles in morphogenesis and cell differentiation. Dev Dyn. 1997;210:383–396. - PubMed
-
- Andl T, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. - PubMed
-
- Begue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci. 1998;106:963–970. - PubMed
-
- Bei M, Kratochwil K, Maas RL. BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development. 2000;127:4711–4718. - PubMed
-
- Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 1998;125:4325–4333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

