The impact of reference pricing of nonsteroidal anti-inflammatory agents on the use and costs of analgesic drugs
- PMID: 16174135
- PMCID: PMC1361214
- DOI: 10.1111/j.1475-6773.2005.00420.x
The impact of reference pricing of nonsteroidal anti-inflammatory agents on the use and costs of analgesic drugs
Abstract
Objective: To estimate the effect of reference pricing (RP) of nonsteroidal anti-inflammatory drugs (NSAIDs) on drug subsidy program and beneficiary expenditures on analgesic drugs.
Data sources/study setting: Monthly claims data from Pharmacare, the public drug subsidy program for seniors in British Columbia, Canada, over the period of February 1993 to June 2001.
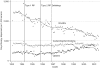
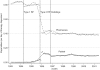
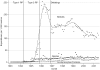
Study design: RP limits drug plan reimbursement of interchangeable medicines to a reference price, which is typically equal to the price of the lowest cost interchangeable drug; any cost above that is borne by the patient. Pharmacare introduced two different forms of RP to the NSAIDs, Type 1 in April 1994 and Type 2 in November 1995. Under Type 1 RP, generic and brand versions of the same NSAID are considered interchangeable, whereas under Type 2 RP different NSAIDs are considered interchangeable. We extrapolated average reimbursement per day of NSAID therapy over the months before RP to estimate what expenditures would have been without the policies. These counterfactual predictions were compared with actual values to estimate the impact of the policies; the estimated impacts on reimbursement rates were multiplied by the postpolicy volume of NSAIDS dispensed, which appeared unaffected by the policies, to estimate expenditure changes.
Principal findings: After Type 2 RP, program expenditures declined by $22.7 million (CAN), or $4 million (CAN), annually cutting expenditure by about half. Most savings accrued from the substitution of low-cost NSAIDs for more costly alternatives. About 20 percent of savings represented expenditures by seniors who elected to pay for partially reimbursed drugs. Type 1 RP produced one-quarter the savings of type 2 RP.
Conclusions: Type 2 RP of NSAIDs achieved its goal of reducing drug expenditures and was more effective than Type 1 RP. The effects of RP on patient health and associated health care costs remain to be investigated.
Figures


Similar articles
-
A re-examination of the impact of reference pricing on anti-hypertensive drug plan expenditures in British Columbia.Health Econ. 2006 Jul;15(7):735-42. doi: 10.1002/hec.1103. Health Econ. 2006. PMID: 16498702
-
Impact of reference-based pricing for histamine-2 receptor antagonists and restricted access for proton pump inhibitors in British Columbia.CMAJ. 2002 Jun 25;166(13):1655-62. CMAJ. 2002. PMID: 12126319 Free PMC article.
-
Effect of a prior-authorization requirement on the use of nonsteroidal antiinflammatory drugs by Medicaid patients.N Engl J Med. 1995 Jun 15;332(24):1612-7. doi: 10.1056/NEJM199506153322406. N Engl J Med. 1995. PMID: 7753141
-
Reference-based pricing in British Columbia: implications for cardiologists--an analysis.Can J Cardiol. 1997 Jan;13(1):46-51. Can J Cardiol. 1997. PMID: 9039064 Review.
-
The economics of prescription drug prices, government intervention, and the importation of drugs from Canada.Nurs Econ. 2005 Nov-Dec;23(6):307-11, 279. Nurs Econ. 2005. PMID: 16459902 Review.
Cited by
-
Pharmaceutical policies: effects of restrictions on reimbursement.Cochrane Database Syst Rev. 2010 Aug 4;2010(8):CD008654. doi: 10.1002/14651858.CD008654. Cochrane Database Syst Rev. 2010. PMID: 20687098 Free PMC article.
-
Effects of reference pricing in pharmaceutical markets: a review.Pharmacoeconomics. 2011 Jan;29(1):17-33. doi: 10.2165/11537860-000000000-00000. Pharmacoeconomics. 2011. PMID: 21142276 Review.
-
Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies.Cochrane Database Syst Rev. 2014 Oct 16;2014(10):CD005979. doi: 10.1002/14651858.CD005979.pub2. Cochrane Database Syst Rev. 2014. PMID: 25318966 Free PMC article.
-
Assessing the impact of global price interdependencies.Pharmacoeconomics. 2008;26(8):649-59. doi: 10.2165/00019053-200826080-00003. Pharmacoeconomics. 2008. PMID: 18620459
-
Reference drug programs: effectiveness and policy implications.Health Policy. 2007 Apr;81(1):17-28. doi: 10.1016/j.healthpol.2006.05.001. Epub 2006 Jun 13. Health Policy. 2007. PMID: 16777256 Free PMC article.
References
-
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines “Recommendations for the Medical Management of Osteoarthritis of the Hip and Knee.”. Arthritis and Rheumatism. 2000;43(9):1905–15. - PubMed
-
- Anis A, Wen Q. “Price Regulation of Pharmaceuticals in Canada.”. Journal of Health Economics. 1998;17:21–38. - PubMed
-
- British Columbia Ministry of Health Services “Pharmacare Trends 2000”. 2001. [accessed on September 14, 2004]. Available at http://www.healthservices.gov.bc.ca/pharme/outgoing/PcareTrends2000.pdf.
-
- Brooks PM, Day RO. “Nonsteroidal Antiinflammatory Drugs—Differences and Similarities.”. New England Journal of Medicine. 1991;324:1716–25. - PubMed
-
- Health Canada . Arthritis in Canada. An Ongoing Challenge (Cat. # H39-4/14-2003E) Ottawa: Health Canada; 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

