Cerebrospinal fluid may mediate CNS ischemic injury
- PMID: 16174300
- PMCID: PMC1253519
- DOI: 10.1186/1743-8454-2-7
Cerebrospinal fluid may mediate CNS ischemic injury
Abstract
Background: The central nervous system (CNS) is extremely vulnerable to ischemic injury. The details underlying this susceptibility are not completely understood. Since the CNS is surrounded by cerebrospinal fluid (CSF) that contains a low concentration of plasma protein, we examined the effect of changing the CSF in the evolution of CNS injury during ischemic insult.
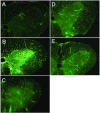
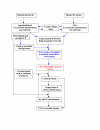
Methods: Lumbar spinal cord ischemia was induced in rabbits by cross-clamping the descending abdominal aorta for 1 h, 2 h or 3 h followed by 7 d of reperfusion. Prior to ischemia, rabbits were subjected to the following procedures; 1) CSF depletion, 2) CSF replenishment at 0 mmHg intracranial pressure (ICP), and 3) replacement of CSF with 8% albumin- or 1% gelatin-modified artificial CSF, respectively. Motor function of the hind limbs and histopathological changes of the spinal cord were scored. Post-ischemic microcirculation of the spinal cord was visualized by fluorescein isothiocyanate (FITC) albumin.
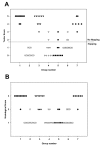
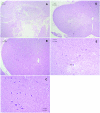
Results: The severity of histopathological damage paralleled the neurological deficit scores. Paraplegia and associated histopathological changes were accompanied by a clear post-ischemic deficit in blood perfusion. Spinal cord ischemia for 1 h resulted in permanent paraplegia in the control group. Depletion of the CSF significantly prevented paraplegia. CSF replenishment with the ICP reduced to 0 mmHg, did not prevent paraplegia. Replacement of CSF with albumin- or gelatin-modified artificial CSF prevented paraplegia in rabbits even when the ICP was maintained at 10-15 mmHg.
Conclusion: We conclude that the presence of normal CSF may contribute to the vulnerability of the spinal cord to ischemic injury. Depletion of the CSF or replacement of the CSF with an albumin- or gelatin-modified artificial CSF can be neuroprotective.
Figures
Similar articles
-
Limiting ischemic spinal cord injury using a free radical scavenger 21-aminosteroid and/or cerebrospinal fluid drainage.J Neurosurg. 1993 Nov;79(5):742-51. doi: 10.3171/jns.1993.79.5.0742. J Neurosurg. 1993. PMID: 8410254
-
Erdosteine ameliorates neurological outcome and oxidative stress due to ischemia/reperfusion injury in rabbit spinal cord.Eur J Vasc Endovasc Surg. 2004 Oct;28(4):379-86. doi: 10.1016/j.ejvs.2004.06.004. Eur J Vasc Endovasc Surg. 2004. PMID: 15350559
-
Prevention of spinal cord ischemia by monitoring spinal cord perfusion pressure and somatosensory evoked potentials.J Cardiovasc Surg (Torino). 1989 Jul-Aug;30(4):565-71. J Cardiovasc Surg (Torino). 1989. PMID: 2777863
-
[Prevention of spinal cord ischemia after cross-clamping of the thoracic aorta--monitoring of spinal cord perfusion pressure and somatosensory evoked potentials].Nihon Kyobu Geka Gakkai Zasshi. 1989 Sep;37(9):1923-31. Nihon Kyobu Geka Gakkai Zasshi. 1989. PMID: 2600466 Japanese.
-
A Brief Overview of the Cerebrospinal Fluid System and Its Implications for Brain and Spinal Cord Diseases.Front Hum Neurosci. 2022 Jan 21;15:737217. doi: 10.3389/fnhum.2021.737217. eCollection 2021. Front Hum Neurosci. 2022. PMID: 35126070 Free PMC article. Review.
Cited by
-
Intrathecal drug delivery in the era of nanomedicine.Adv Drug Deliv Rev. 2020;165-166:77-95. doi: 10.1016/j.addr.2020.02.006. Epub 2020 Mar 3. Adv Drug Deliv Rev. 2020. PMID: 32142739 Free PMC article.
-
Paraplegia after thoracoabdominal aortic surgery: not just assisted circulation, hypothermic arrest, clamp and sew, or TEVAR.Ann Cardiothorac Surg. 2012 Sep;1(3):365-72. doi: 10.3978/j.issn.2225-319X.2012.08.06. Ann Cardiothorac Surg. 2012. PMID: 23977522 Free PMC article. No abstract available.
-
Treatment of rat spinal cord injury with the neurotrophic factor albumin-oleic acid: translational application for paralysis, spasticity and pain.PLoS One. 2011;6(10):e26107. doi: 10.1371/journal.pone.0026107. Epub 2011 Oct 26. PLoS One. 2011. PMID: 22046257 Free PMC article.
References
-
- Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia: WB Saunders Company; 1998.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

