Microtubule movements on the arms of mitotic chromosomes: polar ejection forces quantified in vitro
- PMID: 16174726
- PMCID: PMC1236563
- DOI: 10.1073/pnas.0506017102
Microtubule movements on the arms of mitotic chromosomes: polar ejection forces quantified in vitro
Abstract
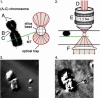
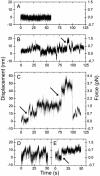
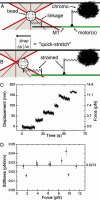
During mitosis, "polar ejection forces" (PEFs) are hypothesized to direct prometaphase chromosome movements by pushing chromosome arms toward the spindle equator. PEFs are postulated to be caused by (i) plus-end-directed microtubule (MT)-based motor proteins on the chromosome arms, namely chromokinesins, and (ii) the polymerization of spindle MTs into the chromosome. However, the exact role of PEFs is unclear, because little is known about their magnitude or their forms (e.g., impulsive vs. sustained, etc.). In this study, we employ optical tweezers to bring about the lateral interaction between chromosome arms and MTs in vitro to directly measure the speed and force of the PEFs developed on chromosome arms. We find that forces are unidirectional and frequently exceed 1 pN, with maximum forces of 2-3 pN and peak velocities of 63 +/- 41 nm/s; the movements are ATP-dependent and exhibit a characteristic noncontinuous motion that includes displacements of >50 nm, stalls, and backwards slippage of the MT even under low loads. We perform experiments using antibodies to the chromokinesins Kid and KIF4 that identify Kid as the principal force-producing agent for PEFs. At first glance, this motor activity appears surprisingly weak and erratic, but it explains how PEFs can guide chromosome movements without severely deforming or damaging the local chromosome structure.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

