Intrathymic administration of hematopoietic progenitor cells enhances T cell reconstitution in ZAP-70 severe combined immunodeficiency
- PMID: 16174749
- PMCID: PMC1224628
- DOI: 10.1073/pnas.0504268102
Intrathymic administration of hematopoietic progenitor cells enhances T cell reconstitution in ZAP-70 severe combined immunodeficiency
Abstract
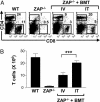
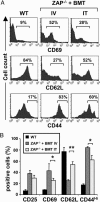
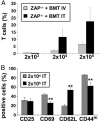
Patients with severe combined immunodeficiency (SCID) present with opportunistic infections that are almost universally fatal in infancy. The mainstay treatment for these patients is allogeneic hematopoietic stem cell (HSC) transplantation, but sustained polyclonal T cell reconstitution is too often unsatisfactory. Although transplantation is conventionally performed by i.v. administration of HSC, we hypothesized that an intrathymic strategy would be superior. Indeed, several progenitor cell populations are incapable of homing to the thymus, the major site of T cell differentiation, and it appears that there are extensive time periods during which the thymus is refractory to progenitor cell import. To test this hypothesis, nonconditioned infant ZAP-70-deficient SCID mice were intrathymically injected with WT bone marrow progenitor cells, a procedure accomplished without surgical intervention. Upon intrathymic HSC injection, there was a more rapid T cell differentiation, with mature thymocytes detected by 4 weeks after transplantation. Intrathymic injection of HSC also resulted in significantly higher numbers of peripheral T cells, increased percentages of naïve T cells, and more diverse T cell receptor repertoires. Moreover, T cell reconstitution after intrathymic transplantation was obtained after injection of 10-fold fewer donor HSC. Thus, this intrathymic transplantation approach may improve the outcome of SCID patients by enhancing T cell reconstitution.
Figures


References
-
- Antoine, C., Muller, S., Cant, A., Cavazzana-Calvo, M., Veys, P., Vossen, J., Fasth, A., Heilmann, C., Wulffraat, N., Seger, R., et al. (2003) Lancet 361, 553-560. - PubMed
-
- Buckley, R. H., Schiff, S. E., Schiff, R. I., Markert, L., Williams, L. W., Roberts, J. L., Myers, L. A. & Ward, F. E. (1999) N. Engl. J. Med. 340, 508-516. - PubMed
-
- Patel, D. D., Gooding, M. E., Parrott, R. E., Curtis, K. M., Haynes, B. F. & Buckley, R. H. (2000) N. Engl. J. Med. 342, 1325-1332. - PubMed
-
- Spangrude, G. J. & Weissman, I. L. (1988) J. Immunol. 141, 1877-1890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

