Cytoskeletal coordination during neuronal migration
- PMID: 16174753
- PMCID: PMC1199551
- DOI: 10.1073/pnas.0506008102
Cytoskeletal coordination during neuronal migration
Abstract
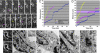
Discoveries from human and mouse genetics have identified cytoskeletal and signaling proteins that are essential for neuronal migration in the developing brain. To provide a meaningful context for these studies, we took an unbiased approach of correlative electron microscopy of neurons migrating through a three-dimensional matrix, and characterized the cytoskeletal events that occur as migrating neurons initiate saltatory forward movements of the cell nucleus. The formation of a cytoplasmic dilation in the proximal leading process precedes nuclear translocation. Cell nuclei translocate into these dilations in saltatory movements. Time-lapse imaging and pharmacological perturbation suggest that nucleokinesis requires stepwise or hierarchical interactions between microtubules, myosin II, and cell adhesion. We hypothesize that these interactions couple leading process extension to nuclear translocation during neuronal migration.
Figures




References
-
- O'Rourke, N. A., Dailey, M. E., Smith, S. J. & McConnell, S. K. (1992) 258, 299-302. - PubMed
-
- O'Rourke, N. A., Sullivan, D. P., Kaznowski, C. E., Jacobs, A. A. & McConnell, S. K. (1995) Development (Cambridge, U.K.) 121, 2165-2176. - PubMed
-
- O'Rourke, N. A., Chenn, A. & McConnell, S. K. (1997) Development (Cambridge, U.K.) 124, 997-1005. - PubMed
-
- Nadarajah, B., Alifragis, P., Wong, R. O. L. & Parnavelas, J. G. (2002) Nat. Neurosci. 5, 218-224. - PubMed
-
- Hatten, M. E. (2002) Science 297, 1660-1663. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

