Second-line treatment with irinotecan plus cisplatin vs cisplatin of patients with advanced non-small-cell lung cancer pretreated with taxanes and gemcitabine: a multicenter randomised phase II study
- PMID: 16175189
- PMCID: PMC2361638
- DOI: 10.1038/sj.bjc.6602748
Second-line treatment with irinotecan plus cisplatin vs cisplatin of patients with advanced non-small-cell lung cancer pretreated with taxanes and gemcitabine: a multicenter randomised phase II study
Abstract
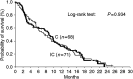
The aim of this study was to compare the irinotecan/cisplatin regimen with cisplatin as second-line chemotherapy in patients with advanced non-small-cell lung cancer (NSCLC) pretreated with a taxane/gemcitabine regimen. Patients (n = 147) with stage IV NSCLC pretreated with a taxane/gemcitabine regimen were randomly assigned to receive either irinotecan (110 mg m(-2), day 1 and 100 mg m(-2), day 8) and cisplatin (80 mg m(-2), day 8) (IC; n = 74) or CDDP (80 mg m(-2), day 1) (C; n = 73) every 3 weeks. Patients treated with IC and C had a median survival of 7.8 and 8.8 months, respectively (P = 0.933). The 1-year survival rate was 34.3% for IC-treated patients and 31.7% for C-treated patients. Cox's regression analysis revealed that response to treatment (hazard ratio (HR) = 2.787; 95% confidence interval (CI): 1.1578-4.922) and performance status (HR = 1.865; 95% CI: 1.199-2.872) was independent prognostic factors for survival. Overall response rate was 22.5% (95% CI: 12.8-32.2%) for IC-treated patients and 7.0% (95% CI: 1.15-13.6%) for C-treated patients (P = 0.012); tumour growth control (partial remission (PR) + stable disease (SD)) was observed in 26 (38%) IC and 25 (36%) C patients (P = 0.878). There was no difference in terms of quality of life between the two chemotherapy arms. The incidence of febrile neutropenia, grade 3 and 4 neutropenia and grade 3 and 4 diarrhoea was significantly higher in the IC- than the C-treated patients. Other toxicities were mild. There were no treatment-related deaths in either arm. The IC regimen did not confer a survival benefit compared with C as second-line treatment of patients with advanced NSCLC pretreated with a taxane/gemcitabine regimen, despite its better efficacy in terms of response rate.
Figures
References
-
- Agelaki S, Bania H, Kouroussis Ch, Blazoyiannakis G, Souglakos J, Tsiafaki X, Kalbakis K, Rapti A, Androulakis N, Georgoulias V, Papadakis E (2001) Second-line treatment with vinorelbine and carboplatin in patients with advanced non-small cell lung cancer. A multicenter phase II study. Lung Cancer 34(Suppl 4): 77–80 - PubMed
-
- Androulakis N, Kouroussis CH, Kakolyris S, Tzannes S, Papadakis E, Papadimitriou C, Geroyianni A, Georgopoulou T, Dimopoulou I, Souglakos J, Kotsakis A, Vardakis N, Hatzidaki D, Georgoulias V (1998) Salvage treatment with paclitaxel and gemcitabine for patients with non-small cell lung cancer after cisplatin- or docetaxel-based chemotherapy: a multicenter phase II study. Ann Oncol 9: 1127–1130 - PubMed
-
- Belani CP (1993) Multimodality management of regionally advanced non-small cell lung cancer. Semin Onco 20: 302–314 - PubMed
-
- Belani CP (1998) Single agents in the second-line treatment of non-small cell lung cancer. Semin Oncol 25(3, Suppl 8): 10–14 - PubMed
-
- Boisseau M, Guichard S, Canal P (1996) Irinotecan (CPT-11): current status and perspectives. Exp Opin Invest Drugs 5: 613–626
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials