Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa
- PMID: 16175511
- PMCID: PMC1275614
- DOI: 10.1086/496901
Loss of desmoplakin tail causes lethal acantholytic epidermolysis bullosa
Abstract
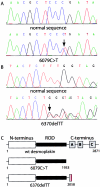
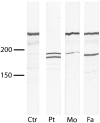
The cytoplasmic plaque protein desmoplakin (DP), which is located in desmosomes, plays a major role in epithelial and muscle cell adhesion by linking the transmembrane cadherins to the cytoplasmic intermediate filament network. Mutations of DP may cause striate palmoplantar keratoderma, arrhythmogenic right ventricular dysplasia, skin fragility/woolly hair syndrome, Naxos-like disease, and Carvajal syndrome. DP must be indispensable, because DP-/- mice are early abortive. Here, we report a patient with severe fragility of skin and mucous membranes caused by genetic truncation of the DP tail. The new phenotype is lethal in the neonatal period because of immense transcutaneous fluid loss. The phenotype also comprised universal alopecia, neonatal teeth, and nail loss. Histology showed suprabasal clefting and acantholysis throughout the spinous layer, mimicking pemphigus. Electron microscopy revealed disconnection of keratin intermediate filaments from desmosomes. Immunofluorescence staining of DP showed a distinct punctate intercellular pattern in the patient's skin. Protein analysis revealed expression of truncated DP polypeptides. Mutational analysis of the patient demonstrated compound heterozygosity for two DP mutations, 6079C-->T (R1934X) and 6370delTT, respectively. Aberrant mRNA transcripts that predict premature termination of translation with loss of the three intermediate filament-binding subdomains in the DP tail were detected by RT-PCR. The new dramatic phenotype, which we named "lethal acantholytic epidermolysis bullosa," underscores the paramount role of DP in epidermal integrity.
Figures



Similar articles
-
Lethal acantholytic epidermolysis bullosa.Dermatol Clin. 2010 Jan;28(1):131-5. doi: 10.1016/j.det.2009.10.015. Dermatol Clin. 2010. PMID: 19945626 Review.
-
Korean Monozygotic Twins with Lethal Acantholytic Epidermolysis Bullosa Caused by Two Novel DSP Mutations.Ann Clin Lab Sci. 2017 Mar;47(2):213-216. Ann Clin Lab Sci. 2017. PMID: 28442525
-
Compound heterozygosity for non-sense and mis-sense mutations in desmoplakin underlies skin fragility/woolly hair syndrome.J Invest Dermatol. 2002 Feb;118(2):232-8. doi: 10.1046/j.0022-202x.2001.01664.x. J Invest Dermatol. 2002. PMID: 11841538
-
Striate palmoplantar keratoderma arising from desmoplakin and desmoglein 1 mutations is associated with contrasting perturbations of desmosomes and the keratin filament network.Br J Dermatol. 2004 May;150(5):878-91. doi: 10.1111/j.1365-2133.2004.05996.x. Br J Dermatol. 2004. PMID: 15149499
-
Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy.Cardiovasc Pathol. 2004 Jul-Aug;13(4):185-94. doi: 10.1016/j.carpath.2004.03.609. Cardiovasc Pathol. 2004. PMID: 15210133 Review.
Cited by
-
Prediction of Recurrence and Survival for Triple-Negative Breast Cancer (TNBC) by a Protein Signature in Tissue Samples.Mol Cell Proteomics. 2015 Nov;14(11):2936-46. doi: 10.1074/mcp.M115.048967. Epub 2015 Jul 24. Mol Cell Proteomics. 2015. PMID: 26209610 Free PMC article.
-
Inherited epidermolysis bullosa.Orphanet J Rare Dis. 2010 May 28;5:12. doi: 10.1186/1750-1172-5-12. Orphanet J Rare Dis. 2010. PMID: 20507631 Free PMC article. Review.
-
A molecular optomechanics approach reveals functional relevance of force transduction across talin and desmoplakin.Sci Adv. 2023 Jun 23;9(25):eadg3347. doi: 10.1126/sciadv.adg3347. Epub 2023 Jun 21. Sci Adv. 2023. PMID: 37343090 Free PMC article.
-
Desmoglein-2: a novel regulator of apoptosis in the intestinal epithelium.Mol Biol Cell. 2007 Nov;18(11):4565-78. doi: 10.1091/mbc.e07-05-0426. Epub 2007 Sep 5. Mol Biol Cell. 2007. PMID: 17804817 Free PMC article.
-
The Diminution of R-Loops Generated by LncRNA DSP-AS1 Inhibits DSP Gene Transcription to Impede the Re-Epithelialization During Diabetic Wound Healing.Adv Sci (Weinh). 2025 Mar;12(12):e2406021. doi: 10.1002/advs.202406021. Epub 2025 Feb 7. Adv Sci (Weinh). 2025. PMID: 39921255 Free PMC article.
References
Web Resources
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for DSP [accession number M77830]
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for desmoplakin-related syndromes)
References
-
- Alcalai R, Metzger S, Rosenheck S, Meiner V, Chajek-Shaul T (2003) A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol 42:319–327 - PubMed
-
- Armstrong DK, McKenna KE, Purkis PE, Green KJ, Eady RA, Leigh IM, Hughes AE (1999) Haploinsufficiency of desmoplakin causes a striate subtype of palmoplantar keratoderma. Hum Mol Genet 8:143–148 - PubMed
-
- Carvajal-Huerta L (1998) Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol 39:418–421 - PubMed
-
- Cheong JE, Wessagowit V, McGrath JA (2005) Molecular abnormalities of the desmosomal protein desmoplakin in human disease. Clin Exp Dermatol 30:261–266 - PubMed
-
- Choi HJ, Park-Snyder S, Pascoe LT, Green KJ, Weis WI (2002) Structures of two intermediate filament-binding fragments of desmoplakin reveal a unique repeat motif structure. Nat Struct Biol 9:612–620 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

