Temperature regulates transcription in the zebrafish circadian clock
- PMID: 16176122
- PMCID: PMC1233578
- DOI: 10.1371/journal.pbio.0030351
Temperature regulates transcription in the zebrafish circadian clock
Abstract
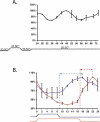
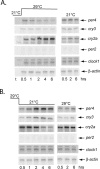
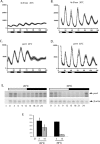
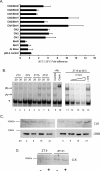
It has been well-documented that temperature influences key aspects of the circadian clock. Temperature cycles entrain the clock, while the period length of the circadian cycle is adjusted so that it remains relatively constant over a wide range of temperatures (temperature compensation). In vertebrates, the molecular basis of these properties is poorly understood. Here, using the zebrafish as an ectothermic model, we demonstrate first that in the absence of light, exposure of embryos and primary cell lines to temperature cycles entrains circadian rhythms of clock gene expression. Temperature steps drive changes in the basal expression of certain clock genes in a gene-specific manner, a mechanism potentially contributing to entrainment. In the case of the per4 gene, while E-box promoter elements mediate circadian clock regulation, they do not direct the temperature-driven changes in transcription. Second, by studying E-box-regulated transcription as a reporter of the core clock mechanism, we reveal that the zebrafish clock is temperature-compensated. In addition, temperature strongly influences the amplitude of circadian transcriptional rhythms during and following entrainment by light-dark cycles, a property that could confer temperature compensation. Finally, we show temperature-dependent changes in the expression levels, phosphorylation, and function of the clock protein, CLK. This suggests a mechanism that could account for changes in the amplitude of the E-box-directed rhythm. Together, our results imply that several key transcriptional regulatory elements at the core of the zebrafish clock respond to temperature.
Figures


References
-
- Sweeney BM, Hastings JW. Effects of temperature upon diurnal rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:87–104. - PubMed
-
- Liu Y, Garceau NY, Loros JJ, Dunlap JC. Thermally regulated translational control of FRQ mediates aspects of temperature responses in the neurospora circadian clock. Cell. 1997;89:477–486. - PubMed
-
- Pittendrigh CS. Temporal organization: Reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

