Neonatal androgenization of hypogonadal (hpg) male mice does not abolish estradiol-induced FSH production and spermatogenesis
- PMID: 16176578
- PMCID: PMC1249589
- DOI: 10.1186/1477-7827-3-48
Neonatal androgenization of hypogonadal (hpg) male mice does not abolish estradiol-induced FSH production and spermatogenesis
Abstract
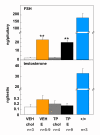
Background: Testicular development is arrested in the hypogonadal (hpg) mouse due to a congenital deficiency in hypothalamic gonadotropin-releasing hormone (GnRH) synthesis. Chronic treatment of male hpg mice with estradiol induces FSH synthesis and secretion, and causes testicular maturation and qualitatively normal spermatogenesis. As estradiol negative feedback normally inhibits FSH production in the male, this study tested whether this paradoxical response to estradiol in the male hpg mouse might be due to inadequate masculinisation or incomplete defeminization in the neonatal period. Previous studies have demonstrated that treatment of hpg mice with testosterone propionate in the immediate neonatal period is necessary to allow full reproductive behaviors to be expressed following suitable endocrine stimulation at adult ages.
Methods: Hpg mice were treated with 100 mug testosterone propionate or vehicle on postnatal day 2. At 35 days of age, subgroups of these mice were treated with silastic implants containing estradiol or cholesterol. Reproductive behavior was scored in tests with steroid-primed female mice, then testicular development was assessed histologically, and measures of pituitary FSH content made at 85 days of age.
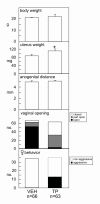
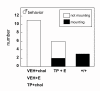
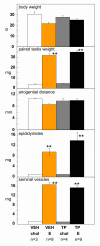
Results: The neonatal testosterone propionate treatment successfully defeminized female litter mates, as revealed by impaired vaginal opening and deficiencies in lordosis behavior, and it allowed appropriate male reproductive behavior to be expressed in a proportion of the hpg males when tested at an adult age. However, neonatal androgen supplementation did not block or even reduce the subsequent actions of estradiol in increasing pituitary FSH content, nor did it affect the ability of estradiol to induce qualitatively normal spermatogenesis.
Conclusion: The ability of the hpg male to show a "female" neuroendocrine response to estradiol is not a result of inadequate androgenization during neonatal development, and thus the actions of estradiol revealed in this rodent model are not an artefact of incomplete sexual differentiation, but reflect a physiological role of estradiol occurring during a specific early temporal window of male reproductive development.
Figures

Similar articles
-
Estrogenic induction of spermatogenesis in the hypogonadal (hpg) mouse: role of androgens.Reproduction. 2005 Nov;130(5):643-54. doi: 10.1530/rep.1.00693. Reproduction. 2005. PMID: 16264094
-
The hypogonadal (hpg) mouse as a model to investigate the estrogenic regulation of spermatogenesis.Hum Fertil (Camb). 2006 Sep;9(3):127-35. doi: 10.1080/14647270500509103. Hum Fertil (Camb). 2006. PMID: 17008264 Review.
-
Estrogenic induction of spermatogenesis in the hypogonadal mouse.Endocrinology. 2000 Aug;141(8):2861-9. doi: 10.1210/endo.141.8.7596. Endocrinology. 2000. PMID: 10919273
-
Neonatal administration of FSH increases Sertoli cell numbers and spermatogenesis in gonadotropin-deficient (hpg) mice.J Endocrinol. 1996 Oct;151(1):37-48. doi: 10.1677/joe.0.1510037. J Endocrinol. 1996. PMID: 8943767
-
Neural grafts and the restoration of pituitary and gonadal function in hypogonadal (HPG) mice.Ann Endocrinol (Paris). 1987;48(5):378-84. Ann Endocrinol (Paris). 1987. PMID: 3124706 Review.
Cited by
-
Neonatal androgen-dependent sex differences in lumbar spinal cord dopamine concentrations and the number of A11 diencephalospinal dopamine neurons.J Comp Neurol. 2010 Jul 1;518(13):2423-36. doi: 10.1002/cne.22340. J Comp Neurol. 2010. PMID: 20503420 Free PMC article.
-
Effects of estradiol and FSH on maturation of the testis in the hypogonadal (hpg) mouse.Reprod Biol Endocrinol. 2008 Jan 29;6:4. doi: 10.1186/1477-7827-6-4. Reprod Biol Endocrinol. 2008. PMID: 18230131 Free PMC article.
-
Hypothalamic control of the male neonatal testosterone surge.Philos Trans R Soc Lond B Biol Sci. 2016 Feb 19;371(1688):20150115. doi: 10.1098/rstb.2015.0115. Epub 2016 Feb 1. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 26833836 Free PMC article. Review.
-
Role of estrogen receptors and g protein-coupled estrogen receptor in regulation of hypothalamus-pituitary-testis axis and spermatogenesis.Front Endocrinol (Lausanne). 2014 Jan 16;5:1. doi: 10.3389/fendo.2014.00001. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 24474947 Free PMC article. Review.
-
Sex differences in β-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure.Brain Res. 2010 Dec 17;1366:233-45. doi: 10.1016/j.brainres.2010.10.009. Epub 2010 Oct 8. Brain Res. 2010. PMID: 20934413 Free PMC article.
References
-
- Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. JCEM. 1999;84:4677–4694. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

