Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength
- PMID: 16176974
- PMCID: PMC1289398
- DOI: 10.1091/mbc.e05-05-0410
Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength
Abstract
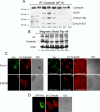
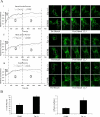
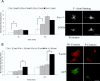
Cortactin regulates the strength of nascent N-cadherin-mediated intercellular adhesions through a tyrosine phosphorylation-dependent mechanism. Currently, the functional significance of cortactin phosphorylation and the kinases responsible for the regulation of adhesion strength are not defined. We show that the nonreceptor tyrosine kinase Fer phosphorylates cadherin-associated cortactin and that this process is involved in mediating intercellular adhesion strength. In wild-type fibroblasts N-cadherin ligation-induced transient phosphorylation of Fer, indicating that junction formation activates Fer kinase. Tyrosine phosphorylation of cortactin after N-cadherin ligation was strongly reduced in fibroblasts expressing only catalytically inactive Fer (D743R), compared with wild-type cells. In wild-type cells, N-cadherin-coated bead pull-off assays induced fourfold greater endogenous N-cadherin association than in D743R cells. Fluorescence recovery after photobleaching showed that GFP-N-cadherin mobility at nascent contacts was 50% faster in wild-type than D743R cells. In shear wash-off assays, nascent intercellular adhesion strength was twofold higher in wild-type than D743R cells. Cortactin recruitment to adhesions was independent of Fer kinase activity, but was impacted by N-cadherin ligation-provoked Rac activation. We conclude that N-cadherin ligation induces Rac-dependent cortactin recruitment and Fer-dependent cortactin phosphorylation, which in turn promotes enhanced mobilization and interaction of surface expressed N-cadherin in contacting cells.
Figures






Similar articles
-
Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts.J Cell Sci. 2004 Oct 1;117(Pt 21):5117-31. doi: 10.1242/jcs.01385. Epub 2004 Sep 21. J Cell Sci. 2004. PMID: 15383621
-
Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion.Mol Cell Biol. 2007 Sep;27(17):6140-52. doi: 10.1128/MCB.01744-06. Epub 2007 Jul 2. Mol Cell Biol. 2007. PMID: 17606629 Free PMC article.
-
Mice devoid of fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation.Mol Cell Biol. 2001 Jan;21(2):603-13. doi: 10.1128/MCB.21.2.603-613.2001. Mol Cell Biol. 2001. PMID: 11134346 Free PMC article.
-
Beyond the epithelium: cadherin function in fibrous connective tissues.FEBS Lett. 2007 Jan 23;581(2):167-74. doi: 10.1016/j.febslet.2006.12.029. Epub 2007 Jan 3. FEBS Lett. 2007. PMID: 17217950 Review.
-
The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin.Curr Opin Cell Biol. 2005 Oct;17(5):459-65. doi: 10.1016/j.ceb.2005.08.009. Curr Opin Cell Biol. 2005. PMID: 16099633 Review.
Cited by
-
Fer kinase regulates cell migration through α-dystroglycan glycosylation.Mol Biol Cell. 2012 Mar;23(5):771-80. doi: 10.1091/mbc.E11-06-0517. Epub 2012 Jan 11. Mol Biol Cell. 2012. PMID: 22238358 Free PMC article.
-
Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts.J Mol Med (Berl). 2007 Jun;85(6):545-54. doi: 10.1007/s00109-007-0198-x. Epub 2007 Apr 11. J Mol Med (Berl). 2007. PMID: 17429596 Review.
-
OMICS Analyses Unraveling Related Gene and Protein-Driven Molecular Mechanisms Underlying PACAP 38-Induced Neurite Outgrowth in PC12 Cells.Int J Mol Sci. 2023 Feb 20;24(4):4169. doi: 10.3390/ijms24044169. Int J Mol Sci. 2023. PMID: 36835581 Free PMC article.
-
Tyrosine phosphatase PTPalpha regulates focal adhesion remodeling through Rac1 activation.Am J Physiol Cell Physiol. 2008 Apr;294(4):C931-44. doi: 10.1152/ajpcell.00359.2007. Epub 2008 Jan 23. Am J Physiol Cell Physiol. 2008. PMID: 18216165 Free PMC article.
-
Cortactin is a functional target of E-cadherin-activated Src family kinases in MCF7 epithelial monolayers.J Biol Chem. 2009 Jul 10;284(28):18913-22. doi: 10.1074/jbc.M109.000307. Epub 2009 May 19. J Biol Chem. 2009. PMID: 19457864 Free PMC article.
References
-
- Ben-Dor, I., Bern, O., Tennenbaum, T., and Nir, U. (1999). Cell cycle-dependent nuclear accumulation of the p94fer tyrosine kinase is regulated by its NH2 terminus and is affected by kinase domain integrity and ATP binding. Cell Growth Differ. 10, 113–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

