Regulated costimulation in the thymus is critical for T cell development: dysregulated CD28 costimulation can bypass the pre-TCR checkpoint
- PMID: 16177059
- PMCID: PMC1343453
- DOI: 10.4049/jimmunol.175.7.4199
Regulated costimulation in the thymus is critical for T cell development: dysregulated CD28 costimulation can bypass the pre-TCR checkpoint
Abstract
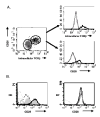
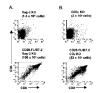
Expression of CD28 is highly regulated during thymic development, with CD28 levels extremely low on immature thymocytes but increasing dramatically as CD4- CD8- cells initiate expression of TCRbeta. B7-1 and B7-2, the ligands for CD28, have a restricted distribution in the thymic cortex where immature thymocytes reside and are more highly expressed in the medulla where the most mature thymocytes are located. To determine the importance of this regulated CD28/B7 expression for T cell development, we examined the effect of induced CD28 signaling of immature thymocytes in CD28/B7-2 double-transgenic mice. Strikingly, we found that differentiation to the CD4+ CD8+ stage in CD28/B7-2 transgenics proceeds independent of the requirement for TCRbeta expression manifest in wild-type thymocytes, occurring even in Rag- or CD3epsilon- knockouts. These findings indicate that signaling of immature thymocytes through CD28 in the absence of TCR- or pre-TCR-derived signals can promote an aberrant pathway of T cell differentiation and highlight the importance of finely regulated physiologic expression of CD28 and B7 in maintaining integrity of the "beta" checkpoint for pre-TCR/TCR-dependent thymic differentiation.
Figures







Similar articles
-
The requirement for pre-TCR during thymic differentiation enforces a developmental pause that is essential for V-DJβ rearrangement.PLoS One. 2011;6(6):e20639. doi: 10.1371/journal.pone.0020639. Epub 2011 Jun 3. PLoS One. 2011. PMID: 21673984 Free PMC article.
-
Maturation versus death of developing double-positive thymocytes reflects competing effects on Bcl-2 expression and can be regulated by the intensity of CD28 costimulation.J Immunol. 2001 Mar 1;166(5):3468-75. doi: 10.4049/jimmunol.166.5.3468. J Immunol. 2001. PMID: 11207305
-
Comparison of thymocyte development and cytokine production in CD7-deficient, CD28-deficient and CD7/CD28 double-deficient mice.Int Immunol. 2001 Feb;13(2):157-66. doi: 10.1093/intimm/13.2.157. Int Immunol. 2001. PMID: 11157849
-
Potential roles of the B7 and CD28 receptor families in autoimmunity and immune evasion.Clin Immunol Immunopathol. 1995 May;75(2):99-111. doi: 10.1006/clin.1995.1058. Clin Immunol Immunopathol. 1995. PMID: 7535672 Review.
-
The T cell surface protein, CD28.Int J Biochem Cell Biol. 1997 Aug-Sep;29(8-9):1053-7. doi: 10.1016/s1357-2725(97)00012-5. Int J Biochem Cell Biol. 1997. PMID: 9416000 Review.
Cited by
-
Maturation of thymocytes with a monoclonal TCR under control of Trac promoter elements in the absence of β-selection.Immunohorizons. 2025 Jul 15;9(8):vlaf035. doi: 10.1093/immhor/vlaf035. Immunohorizons. 2025. PMID: 40664176 Free PMC article.
-
ATM influences the efficiency of TCRβ rearrangement, subsequent TCRβ-dependent T cell development, and generation of the pre-selection TCRβ CDR3 repertoire.PLoS One. 2013 Apr 23;8(4):e62188. doi: 10.1371/journal.pone.0062188. Print 2013. PLoS One. 2013. PMID: 23626787 Free PMC article.
-
γδ T cells acquire effector fates in the thymus and differentiate into cytokine-producing effectors in a Listeria model of infection independently of CD28 costimulation.PLoS One. 2013 May 9;8(5):e63178. doi: 10.1371/journal.pone.0063178. Print 2013. PLoS One. 2013. PMID: 23671671 Free PMC article.
-
ATM deficiency impairs thymocyte maturation because of defective resolution of T cell receptor alpha locus coding end breaks.Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6323-8. doi: 10.1073/pnas.0611222104. Epub 2007 Apr 3. Proc Natl Acad Sci U S A. 2007. PMID: 17405860 Free PMC article.
-
Stepwise progression of β-selection during T cell development involves histone deacetylation.Life Sci Alliance. 2022 Oct 25;6(1):e202201645. doi: 10.26508/lsa.202201645. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36283704 Free PMC article.
References
-
- Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380. - PubMed
-
- Zheng X, Gao JX, Chang X, Wang Y, Liu Y, Wen J, Zhang H, Zhang J, Zheng P. B7-CD28 interaction promotes proliferation and survival but suppresses differentiation of CD4-CD8- T cells in the thymus. J Immunol. 2004;173:2253. - PubMed
-
- Inaba K, Inaba M, Witmer-Pack M, Hatchcock K, Hodes R, Steinman RM. Expression of B7 costimulator molecules on mouse dendritic cells. Adv Exp Med Biol. 1995;378:65. - PubMed
-
- Nelson AJ, Hosier S, Brady W, Linsley PS, Farr AG. Medullary thymic epithelium expresses a ligand for CTLA4 in situ and in vitro. J Immunol. 1993;151:2453. - PubMed
-
- Harada Y, Tokushima M, Matsumoto Y, Ogawa S, Otsuka M, Hayashi K, Weiss BD, June CH, Abe R. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

