Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins
- PMID: 16177291
- PMCID: PMC1230961
- DOI: 10.1128/IAI.73.10.6199-6209.2005
Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins
Figures
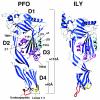
References
-
- Abel, K., M. D. Yoder, R. Hilgenfeld, and F. Jurnak. 1996. An alpha to beta conformational switch in EF-Tu. Structure 4:1153-1159. - PubMed
-
- Abrami, L., M. Fivaz, E. Decroly, N. G. Seidah, F. Jean, G. Thomas, S. H. Leppla, J. T. Buckley, and F. G. van der Goot. 1998. The pore-forming toxin proaerolysin is activated by furin. J. Biol. Chem. 273:32656-32661. - PubMed
-
- Alving, C. R., W. H. Habig, K. A. Urban, and M. C. Hardegree. 1979. Cholesterol-dependent tetanolysin damage to liposomes. Biochim. Biophys. Acta 551:224-228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

