Inhibition of cell surface export of group A streptococcal anchorless surface dehydrogenase affects bacterial adherence and antiphagocytic properties
- PMID: 16177295
- PMCID: PMC1230963
- DOI: 10.1128/IAI.73.10.6237-6248.2005
Inhibition of cell surface export of group A streptococcal anchorless surface dehydrogenase affects bacterial adherence and antiphagocytic properties
Abstract
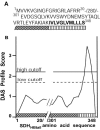
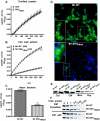
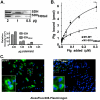
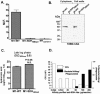
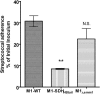
Surface dehydrogenase (SDH) is an anchorless, multifunctional protein displayed on the surfaces of group A Streptococcus (GAS) organisms. SDH is encoded by a single gene, sdh (gap or plr) that is essential for bacterial survival. Hence, the resulting nonfeasibility of creating a knockout mutant is a major limiting factor in studying its role in GAS pathogenesis. An insertion mutagenesis strategy was devised in which a nucleotide sequence encoding a hydrophobic tail of 12 amino acids ((337)IVLVGLVMLLLS(348)) was added at the 3' end of the sdh gene, successfully creating a viable mutant strain (M1-SDH(HBtail)). In this mutant strain, the SDH(HBtail) protein was not secreted in the medium but was retained in the cytoplasm and to some extent trapped within the cell wall. Hence, SDH(HBtail) was not displayed on the GAS surface. The mutant strain, M1-SDH(HBtail), grew at the same rate as the wild-type strain. The SDH(HBtail) protein displayed the same GAPDH activity as the wild-type SDH protein. Although the whole-cell extracts of the wild-type and mutant strains showed similar GAPDH activities, cell wall extracts of the mutant strain showed 5.5-fold less GAPDH activity than the wild-type strain. The mutant strain, M1-SDH(HBtail), bound significantly less human plasminogen, adhered poorly to human pharyngeal cells, and lost its innate antiphagocytic activity. These results indicate that the prevention of the cell surface export of SDH affects the virulence properties of GAS. The anchorless SDH protein, thus, is an important virulence factor.
Figures

Similar articles
-
Role of extracellular GAPDH in Streptococcus pyogenes virulence.Mo Med. 2013 May-Jun;110(3):236-40. Mo Med. 2013. PMID: 23829111 Free PMC article. Review.
-
Surface export of GAPDH/SDH, a glycolytic enzyme, is essential for Streptococcus pyogenes virulence.mBio. 2011 May 31;2(3):e00068-11. doi: 10.1128/mBio.00068-11. Print 2011. mBio. 2011. PMID: 21628503 Free PMC article.
-
Group A streptococcal surface GAPDH, SDH, recognizes uPAR/CD87 as its receptor on the human pharyngeal cell and mediates bacterial adherence to host cells.J Mol Biol. 2005 Jul 1;350(1):27-41. doi: 10.1016/j.jmb.2005.04.063. J Mol Biol. 2005. PMID: 15922359
-
Multifunctional glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pyogenes is essential for evasion from neutrophils.J Biol Chem. 2006 May 19;281(20):14215-23. doi: 10.1074/jbc.M513408200. Epub 2006 Mar 24. J Biol Chem. 2006. PMID: 16565520
-
[Mechanisms of glyceraldehyde 3-phosphosphate dehydrogenaseis in bacteria adhesion - A review].Wei Sheng Wu Xue Bao. 2016 Sep;56(9):1398-1405. Wei Sheng Wu Xue Bao. 2016. PMID: 29738212 Review. Chinese.
Cited by
-
Mammalian Neuropeptides as Modulators of Microbial Infections: Their Dual Role in Defense versus Virulence and Pathogenesis.Int J Mol Sci. 2021 Apr 1;22(7):3658. doi: 10.3390/ijms22073658. Int J Mol Sci. 2021. PMID: 33915818 Free PMC article. Review.
-
Characterization of Mycoplasma gallisepticum pyruvate dehydrogenase alpha and beta subunits and their roles in cytoadherence.PLoS One. 2018 Dec 10;13(12):e0208745. doi: 10.1371/journal.pone.0208745. eCollection 2018. PLoS One. 2018. PMID: 30532176 Free PMC article.
-
Role of extracellular GAPDH in Streptococcus pyogenes virulence.Mo Med. 2013 May-Jun;110(3):236-40. Mo Med. 2013. PMID: 23829111 Free PMC article. Review.
-
Identification of GAPDH on the surface of Plasmodium sporozoites as a new candidate for targeting malaria liver invasion.J Exp Med. 2016 Sep 19;213(10):2099-112. doi: 10.1084/jem.20160059. Epub 2016 Aug 22. J Exp Med. 2016. PMID: 27551151 Free PMC article.
-
Glutamine synthetase and glucose-6-phosphate isomerase are adhesive moonlighting proteins of Lactobacillus crispatus released by epithelial cathelicidin LL-37.J Bacteriol. 2012 May;194(10):2509-19. doi: 10.1128/JB.06704-11. Epub 2012 Mar 2. J Bacteriol. 2012. PMID: 22389474 Free PMC article.
References
-
- Banks, D. J., S. F. Porcella, K. D. Barbian, S. B. Beres, L. E. Philips, J. M. Voyich, F. R. DeLeo, J. M. Martin, G. A. Somerville, and J. M. Musser. 2004. Progress toward characterization of the group A Streptococcus metagenome: complete genome sequence of a macrolide-resistant serotype M6 strain. J. Infect. Dis. 190:727-738. - PubMed
-
- Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M.-Y. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, D. S. Campbell, T. M. Smith, J. K. McCormick, D. Y. M. Leung, P. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. - PMC - PubMed
-
- Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. Alpha-enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1273-1287. - PubMed
-
- Biesecker, G., J. I. Harris, J. C. Thierry, J. E. Walker, and A. J. Wonacott. 1977. Sequence and structure of d-glyceraldehyde-3-phosphate dehydrogenase from Bacillus stearothermophilus. Nature 266:328-333. - PubMed
-
- Bisno, A. L., and D. L. Stevens. 1996. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 334:240-245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

