Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis
- PMID: 16177308
- PMCID: PMC1230936
- DOI: 10.1128/IAI.73.10.6372-6382.2005
Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis
Abstract
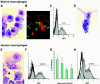
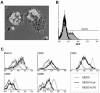
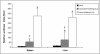
To date, there are no proven vaccines against any form of leishmaniasis. The development of live attenuated vectors shows promise in the field of Leishmania vaccination because these organisms mimic more effectively the course of real infections and can elicit potent activation of the immune system. In the present study, we investigated the potential of a parasitic protozoan that is nonpathogenic to humans, Leishmania tarentolae, as a live candidate vaccine that efficiently targets dendritic cells and lymphoid organs, thus enhancing antigen presentation and consequently influencing the magnitude and quality of T-cell immune responses. We demonstrated that L. tarentolae activates the dendritic cell maturation process and induces T-cell proliferation and the production of gamma interferon, thus skewing CD4(+) T cells toward a Th1 cell phenotype. More importantly, we found that a single intraperitoneal injection of L. tarentolae could elicit a protective immune response against infectious challenge with Leishmania donovani in susceptible BALB/c mice. These results suggest that the use of L. tarentolae as a live vaccine vector may represent a promising approach for improving the effectiveness and safety of candidate live vaccines against Leishmania infections and possibly other intracellular pathogens for which T-cell mediated responses are critical for the development of protective immunity.
Figures

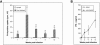
References
-
- Abdelhak, S., H. Louzir, J. Timm, L. Blel, Z. Benlasfar, M. Lagranderie, M. Gheorghiu, K. Dellagi, and B. Gicquel. 1995. Recombinant BCG expressing the leishmania surface antigen Gp63 induces protective immunity against Leishmania major infection in BALB/c mice. Microbiology 141:1585-1592. - PubMed
-
- Alexander, J., G. H. Coombs, and J. C. Mottram. 1998. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 161:6794-6801. - PubMed
-
- Ashford, R. W. 2001. Current usage of nomenclature for parasitic diseases, with special reference to those involving arthropods. Med. Vet. Entomol. 15:121-125. - PubMed
-
- Athman, R., and D. Philpott. 2004. Innate immunity via Toll-like receptors and Nod proteins. Curr. Opin. Microbiol. 7:25-32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

