Anti-idiotypic antibody as a potential candidate vaccine for Neisseria meningitidis serogroup B
- PMID: 16177311
- PMCID: PMC1230893
- DOI: 10.1128/IAI.73.10.6399-6406.2005
Anti-idiotypic antibody as a potential candidate vaccine for Neisseria meningitidis serogroup B
Abstract
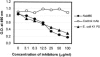
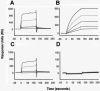
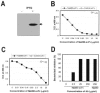
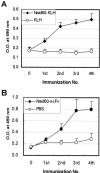
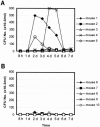
Sepsis and meningitis caused by Neisseria meningitidis serogroup B (NMGB) are serious diseases in infants and young adults, but no effective vaccine is available. The capsular polysaccharide (PS) of NMGB has poor immunogenicity and a structural similarity to polysialic acid (PSA) on neuronal tissue that may elicit autoantibodies. Using HmenB3, a protective and nonautoreactive monoclonal antibody (MAb) to NMGB capsular PS, we produced an anti-idiotypic MAb, Naid60, which mimics the capsular PS of NMGB. We produced an anti-anti-idiotypic MAb, MoB34, by using the immunogenic site on Naid60 responsible for inducing the anti-NMGB PS antibody response. MoB34 elicited the complement-mediated killing of representative strains of serogroup B meningococci. MoB34 did not bind to CHP-134, a neuroblastoma cell line expressing alpha(2-8) PSA, or to mouse brain cryosections at a high concentration. Naid60-keyhole limpet hemocyanin immunization inhibited the growth of live NMGB in intraperitoneally challenged mice; in contrast, three of five control mice developed bacteremia. Thus, Naid60 has an immunogenic site that elicits antibodies with bactericidal activity against NMGB and no autoimmunity to PSA. We suggest that the immunogenic region of Naid60 is a candidate for the development of a new vaccine against NMGB.
Figures


References
-
- Ala'Aldeen, D. D. A., and K. A. V. Cartwright. 1996. Neisseria meningitidis: vaccines and vaccine candidates. J. Infect. 33:153-157. - PubMed
-
- Ashton, F. E., J. A. Ryan, F. Michon, and H. J. Jennings. 1989. Protective efficacy of mouse serum to the N-propionyl derivative of meningococcal group B polysaccharide. Microb. Pathog. 6:455-458. - PubMed
-
- Beninati, C., S. Arseni, G. Mancuso, W. Magliani, S. Conti, A. Midiri, C. Biondo, L. Polonelli, and G. Teti. 2004. Protective immunization against group B meningococci using anti-idiotypic mimics of the capsular polysaccharide. J. Immunol. 172:2461-2468. - PubMed
-
- Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, E. Rosenqvist, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. - PubMed
-
- Bruge, J., N. Bouveret-Le Cam, B. Danve, G. Rougon, and D. Schulz. 2004. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine 22:1087-1096. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

