Role of an alginate lyase for alginate transport in mucoid Pseudomonas aeruginosa
- PMID: 16177314
- PMCID: PMC1230907
- DOI: 10.1128/IAI.73.10.6429-6436.2005
Role of an alginate lyase for alginate transport in mucoid Pseudomonas aeruginosa
Abstract
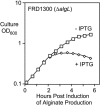
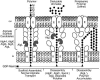
The opportunistic pathogen Pseudomonas aeruginosa secretes a capsule-like polysaccharide called alginate that is important for evasion of host defenses, especially during chronic pulmonary disease of patients with cystic fibrosis (CF). Most proteins for alginate biosynthesis are encoded by the 12-gene algD operon. Interestingly, this operon also encodes AlgL, a lyase that degrades alginate. Mutants lacking AlgG, AlgK, or AlgX, also encoded by the operon, synthesize alginate polymers that are digested by the coregulated protein AlgL. We examined the phenotype of an DeltaalgL mutation in the highly mucoid CF isolate FRD1. Generating a true DeltaalgL mutant was possible only when the algD operon was under the control of a LacI(q)-repressed trc promoter. Upon induction of alginate production with isopropyl-beta-D-thiogalactopyranoside, the DeltaalgL mutant cells were lysed within a few hours. Electron micrographs of the DeltaalgL mutant showed that alginate polymers accumulated in the periplasm, which ultimately burst the bacterial cell wall. The requirement of AlgL in an alginate-overproducing strain led to a new model for alginate secretion in which a multiprotein secretion complex (or scaffold, that includes AlgG, AlgK, AlgX, and AlgL) guides new polymers through the periplasm for secretion across the outer membrane. In this model, AlgL is bifunctional with a structural role in the scaffold and a role in degrading free alginate polymers in the periplasm.
Figures




Similar articles
-
The dual roles of AlgG in C-5-epimerization and secretion of alginate polymers in Pseudomonas aeruginosa.Mol Microbiol. 2003 Feb;47(4):1123-33. doi: 10.1046/j.1365-2958.2003.03361.x. Mol Microbiol. 2003. PMID: 12581364
-
Alginate synthesis in Pseudomonas aeruginosa: the role of AlgL (alginate lyase) and AlgX.J Bacteriol. 1996 Feb;178(3):625-32. doi: 10.1128/jb.178.3.625-632.1996. J Bacteriol. 1996. PMID: 8550492 Free PMC article.
-
Role of the Pseudomonas fluorescens alginate lyase (AlgL) in clearing the periplasm of alginates not exported to the extracellular environment.J Bacteriol. 2005 Dec;187(24):8375-84. doi: 10.1128/JB.187.24.8375-8384.2005. J Bacteriol. 2005. PMID: 16321942 Free PMC article.
-
Mucoid Pseudomonas aeruginosa and cystic fibrosis: the role of mutations in muc loci.FEMS Microbiol Lett. 1992 Dec 15;100(1-3):323-9. doi: 10.1111/j.1574-6968.1992.tb14059.x. FEMS Microbiol Lett. 1992. PMID: 1478467 Review.
-
Pseudomonas aeruginosa: genes and enzymes of alginate synthesis.Trends Microbiol. 1994 May;2(5):151-7. doi: 10.1016/0966-842x(94)90664-5. Trends Microbiol. 1994. PMID: 8055178 Review.
Cited by
-
Alginate-modifying enzymes: biological roles and biotechnological uses.Front Microbiol. 2015 May 27;6:523. doi: 10.3389/fmicb.2015.00523. eCollection 2015. Front Microbiol. 2015. PMID: 26074905 Free PMC article. Review.
-
Biochemical and computational study of an alginate lyase produced by Pseudomonas aeruginosa strain S21.Iran J Basic Med Sci. 2020 Apr;23(4):454-460. doi: 10.22038/ijbms.2020.37277.8874. Iran J Basic Med Sci. 2020. PMID: 32489560 Free PMC article.
-
Biochemical and structural analyses of a bacterial endo-β-1,2-glucanase reveal a new glycoside hydrolase family.J Biol Chem. 2017 May 5;292(18):7487-7506. doi: 10.1074/jbc.M116.762724. Epub 2017 Mar 7. J Biol Chem. 2017. PMID: 28270506 Free PMC article.
-
Two-way AIC: detection of differentially expressed genes from large scale microarray meta-dataset.BMC Genomics. 2013;14 Suppl 2(Suppl 2):S9. doi: 10.1186/1471-2164-14-S2-S9. Epub 2013 Feb 15. BMC Genomics. 2013. PMID: 23445621 Free PMC article.
-
Candidate genes involved in biosynthesis and degradation of the main extracellular matrix polysaccharides of brown algae and their probable evolutionary history.BMC Genomics. 2024 Oct 10;25(1):950. doi: 10.1186/s12864-024-10811-3. BMC Genomics. 2024. PMID: 39390408 Free PMC article.
References
-
- Baltimore, R. S., and M. Mitchell. 1982. Immunologic investigations of mucoid strains of Pseudomonas aeruginosa: comparison of susceptibility to opsonic antibody in mucoid and nonmucoid strains. J. Infect. Dis. 141:238-247. - PubMed
-
- Boyd, A., M. Ghosh, T. B. May, D. Shinabarger, R. Keogh, and A. M. Chakrabarty. 1993. Sequence of the algL gene of Pseudomonas aeruginosa and purification of its alginate lyase product. Gene 131:1-8. - PubMed
-
- Chitnis, C. E., and D. E. Ohman. 1993. Genetic analysis of the alginate biosynthetic gene cluster of Pseudomonas aeruginosa shows evidence of an operonic structure. Mol. Microbiol. 8:583-590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

