Adherence to human vaginal epithelial cells signals for increased expression of Trichomonas vaginalis genes
- PMID: 16177319
- PMCID: PMC1230950
- DOI: 10.1128/IAI.73.10.6472-6478.2005
Adherence to human vaginal epithelial cells signals for increased expression of Trichomonas vaginalis genes
Abstract
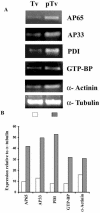
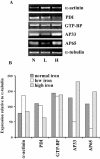
Host parasitism by Trichomonas vaginalis is complex, and the adhesion to vaginal epithelial cells (VECs) by trichomonads is preparatory to colonization of the vagina. Since we showed increased synthesis of adhesins after contact with VECs (A. F. Garcia, et al., Mol. Microbiol. 47:1207-1224, 2003) and more recently demonstrated up-regulated gene expression in VECs after parasite attachment (A. S. Kucknoor, et al., Cell. Microbiol. 7:887-897, 2005), we hypothesized that enhanced expression of adhesin and other genes would result from signaling of trichomonads following adherence. In order to identify the genes that are up-regulated, we constructed a subtraction cDNA library enriched for differentially expressed genes from the parasites that were in contact with the host cells. Thirty randomly selected cDNA clones representing the differentially regulated genes upon initial contact of parasites with host cells were sequenced. Several genes encoded functional proteins with specific functions known to be associated with colonization, such as adherence, change in morphology, and gene transcription and translation. Interestingly, genes unique to trichomonads with unknown functions were also up-regulated. Semiquantitative reverse transcription-PCR (RT-PCR) confirmed expression of select genes. An increased amount of protein was demonstrated by immunoblotting with monoclonal antibody. Finally, we showed the transcriptional regulation of some genes by iron by using RT-PCR. To our knowledge, this is the first report addressing the differential regulation of T. vaginalis genes immediately upon contact with VECs.
Figures

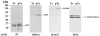
References
-
- Alderete, J. F., J. Nguyen, V. Mundodi, and M. W. Lehker. 2004. Heme-iron increases levels of AP65-mediated adherence by Trichomonas vaginalis. Microb. Pathog. 36:263-271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

