Activation and mitogen-activated protein kinase regulation of transcription factors Ets and NF-kappaB in Mycobacterium-infected macrophages and role of these factors in tumor necrosis factor alpha and nitric oxide synthase 2 promoter function
- PMID: 16177323
- PMCID: PMC1230939
- DOI: 10.1128/IAI.73.10.6499-6507.2005
Activation and mitogen-activated protein kinase regulation of transcription factors Ets and NF-kappaB in Mycobacterium-infected macrophages and role of these factors in tumor necrosis factor alpha and nitric oxide synthase 2 promoter function
Abstract
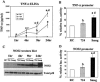
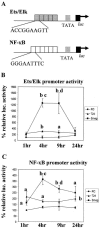
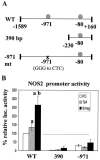
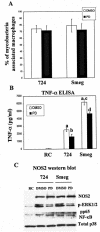
Previous studies have shown that primary murine macrophages infected with Mycobacterium avium produced lower levels of tumor necrosis factor alpha (TNF-alpha) and inducible nitric oxide synthase 2 (NOS2) compared to cells infected with nonpathogenic Mycobacterium smegmatis. TNF-alpha and NOS2 levels correlated with and were dependent on the activation of mitogen-activated protein kinases (MAPKs) p38 and extracellular signal-regulated kinase 1/2 (ERK1/2). To define the macrophage transcriptional responses dependent on ERK1/2 activation following a mycobacterial infection, we used RAW 264.7 cells transfected with a TNF-alpha or NOS2 promoter vector. We determined that macrophages infected with M. avium compared to M. smegmatis showed diminished TNF-alpha and NOS2 promoter activity. A more pronounced difference in promoter activity was observed when only the consensus ETS and NF-kappaB binding sites were used as promoters. Mutational analysis of the ETS and NF-kappaB binding sites present on the TNF-alpha and NOS2 promoters, respectively, showed that these sites were essential for a functional promoter. Moreover, the Ets/Elk but not the NF-kappaB transcriptional response was dependent on ERK1/2. This correlated with the requirement for ERK1/2 in TNF-alpha but not NOS2 promoter activity. Our data indicate that the increased Ets/Elk and NF-kappaB promoter activities associated with M. smegmatis-infected macrophages are responsible, at least in part, for the increased TNF-alpha and NOS2 production observed in these infected cells and that ERK1/2 is required for Ets/Elk activity and full TNF-alpha production.
Figures



Similar articles
-
Differential activation of the transcription factor cyclic AMP response element binding protein (CREB) in macrophages following infection with pathogenic and nonpathogenic mycobacteria and role for CREB in tumor necrosis factor alpha production.Infect Immun. 2005 Jan;73(1):514-22. doi: 10.1128/IAI.73.1.514-522.2005. Infect Immun. 2005. PMID: 15618191 Free PMC article.
-
Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways.J Immunol. 2004 May 1;172(9):5588-97. doi: 10.4049/jimmunol.172.9.5588. J Immunol. 2004. PMID: 15100302
-
Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria.Infect Immun. 2002 Jun;70(6):3040-52. doi: 10.1128/IAI.70.6.3040-3052.2002. Infect Immun. 2002. PMID: 12010996 Free PMC article.
-
Macrophage's proinflammatory response to a mycobacterial infection is dependent on sphingosine kinase-mediated activation of phosphatidylinositol phospholipase C, protein kinase C, ERK1/2, and phosphatidylinositol 3-kinase.J Immunol. 2006 May 1;176(9):5494-503. doi: 10.4049/jimmunol.176.9.5494. J Immunol. 2006. PMID: 16622018
-
Macrophage NOS2 in Tumor Leukocytes.Antioxid Redox Signal. 2017 Jun 20;26(18):1023-1043. doi: 10.1089/ars.2016.6811. Epub 2016 Aug 10. Antioxid Redox Signal. 2017. PMID: 27397579 Review.
Cited by
-
Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression.J Clin Invest. 2009 Dec;119(12):3739-51. doi: 10.1172/JCI39335. Epub 2009 Nov 9. J Clin Invest. 2009. PMID: 19907077 Free PMC article.
-
Mycobacteria bypass mucosal NF-kB signalling to induce an epithelial anti-inflammatory IL-22 and IL-10 response.PLoS One. 2014 Jan 28;9(1):e86466. doi: 10.1371/journal.pone.0086466. eCollection 2014. PLoS One. 2014. PMID: 24489729 Free PMC article.
-
Leishmania major inhibits IL-12 in macrophages by signalling through CR3 (CD11b/CD18) and down-regulation of ETS-mediated transcription.Parasite Immunol. 2013 Dec;35(12):409-20. doi: 10.1111/pim.12049. Parasite Immunol. 2013. PMID: 23834512 Free PMC article.
-
Mycobacterium tuberculosis PE_PGRS17 promotes the death of host cell and cytokines secretion via Erk kinase accompanying with enhanced survival of recombinant Mycobacterium smegmatis.J Interferon Cytokine Res. 2013 Aug;33(8):452-8. doi: 10.1089/jir.2012.0083. Epub 2013 May 10. J Interferon Cytokine Res. 2013. PMID: 23663047 Free PMC article.
-
Pioglitazone, PPARγ agonist, attenuates experimental autoimmune neuritis.Inflammation. 2012 Aug;35(4):1338-47. doi: 10.1007/s10753-012-9447-4. Inflammation. 2012. PMID: 22389055
References
-
- Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. - PubMed
-
- Barthel, R., A. V. Tsytsykova, A. K. Barczak, E. Y. Tsai, C. C. Dascher, M. B. Brenner, and A. E. Goldfeld. 2003. Regulation of tumor necrosis factor alpha gene expression by mycobacteria involves the assembly of a unique enhanceosome dependent on the coactivator proteins CBP/p300. Mol. Cell. Biol. 23:526-533. - PMC - PubMed
-
- Bermudez, L. E., and L. S. Young. 1988. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J. Immunol. 140:3006-3013. - PubMed
-
- Chan, E. D., K. R. Morris, J. T. Belisle, P. Hill, L. K. Remigio, P. J. Brennan, and D. W. Riches. 2001. Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-κB signaling pathways. Infect. Immun. 69:2001-2010. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

