An anthrax lethal factor-neutralizing monoclonal antibody protects rats before and after challenge with anthrax toxin
- PMID: 16177329
- PMCID: PMC1230968
- DOI: 10.1128/IAI.73.10.6547-6551.2005
An anthrax lethal factor-neutralizing monoclonal antibody protects rats before and after challenge with anthrax toxin
Abstract
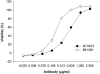
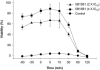
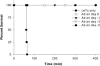
Lethal factor (LF) is a component of anthrax lethal toxin (LeTx). We generated anti-LF murine monoclonal antibodies (MAbs) that show LeTx-neutralizing activity in vitro and in vivo. Anti-LF MAbs were generated by immunization with recombinant LF, and the MAbs showing LeTx-neutralizing activity in vitro were selected. Two MAbs with the highest affinities, 5B13B1 (dissociation constant [K(d)], 2.62 nM) and 3C16C3 (K(d), 8.18 nM), were shown to recognize the same or closely overlapping epitopes on domain III of LF. The 50% inhibitory concentration of 5B13B1 (0.21 microg/ml) was approximately one-third that of 3C16C3 (0.63 microg/ml) in the in vitro LeTx-neutralization assay. The 5B13B1 antibody, which had the highest neutralizing activity, provided perfect protection against LeTx challenge in an in vivo LeTx neutralization assay using Fisher 344 rats. In addition, the antibody showed pre- and postexposure prophylactic effects in the animal experiments. This is the first report that an MAb binding to domain III of LF has neutralizing activity against LeTx. The 5B13B1 antibody may be useful in prophylaxis against anthrax poisoning.
Figures




Similar articles
-
In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity.Infect Immun. 2007 Nov;75(11):5443-52. doi: 10.1128/IAI.00529-07. Epub 2007 Aug 20. Infect Immun. 2007. PMID: 17709410 Free PMC article.
-
The major neutralizing antibody responses to recombinant anthrax lethal and edema factors are directed to non-cross-reactive epitopes.Infect Immun. 2009 Nov;77(11):4714-23. doi: 10.1128/IAI.00749-09. Epub 2009 Aug 31. Infect Immun. 2009. PMID: 19720758 Free PMC article.
-
Monoclonal antibodies directed against protective antigen of Bacillus anthracis enhance lethal toxin activity in vivo.FEMS Immunol Med Microbiol. 2011 Jun;62(1):11-22. doi: 10.1111/j.1574-695X.2011.00782.x. Epub 2011 Feb 2. FEMS Immunol Med Microbiol. 2011. PMID: 21231965
-
Monoclonal antibody therapies against anthrax.Toxins (Basel). 2011 Aug;3(8):1004-19. doi: 10.3390/toxins3081004. Epub 2011 Aug 15. Toxins (Basel). 2011. PMID: 22069754 Free PMC article. Review.
-
Low molecular weight inhibitors of the protease anthrax lethal factor.Mini Rev Med Chem. 2008 Mar;8(3):290-306. doi: 10.2174/138955708783744083. Mini Rev Med Chem. 2008. PMID: 18336349 Review.
Cited by
-
Characterization of the adaptive immune response of donors receiving live anthrax vaccine.PLoS One. 2021 Dec 20;16(12):e0260202. doi: 10.1371/journal.pone.0260202. eCollection 2021. PLoS One. 2021. PMID: 34928976 Free PMC article.
-
Novel chimpanzee/human monoclonal antibodies that neutralize anthrax lethal factor, and evidence for possible synergy with anti-protective antigen antibody.Infect Immun. 2009 Sep;77(9):3902-8. doi: 10.1128/IAI.00200-09. Epub 2009 Jun 15. Infect Immun. 2009. PMID: 19528217 Free PMC article.
-
Anti-toxin antibodies in prophylaxis and treatment of inhalation anthrax.Future Microbiol. 2009 Feb;4(1):35-43. doi: 10.2217/17460913.4.1.35. Future Microbiol. 2009. PMID: 19207098 Free PMC article. Review.
-
Effects of metalloprotease anthrax lethal factor on its peptide-based inhibitor R9LF-1.Mol Cell Biochem. 2015 Aug;406(1-2):293-9. doi: 10.1007/s11010-015-2447-6. Epub 2015 May 16. Mol Cell Biochem. 2015. PMID: 25981534
-
Combinations of monoclonal antibodies to anthrax toxin manifest new properties in neutralization assays.Infect Immun. 2013 Jun;81(6):1880-8. doi: 10.1128/IAI.01328-12. Epub 2013 Mar 18. Infect Immun. 2013. PMID: 23509144 Free PMC article.
References
-
- Agrawal, A., J. Lingappa, S. H. Leppla, S. Agrawal, A. Jabbar, C. Quinn, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424:329-334. - PubMed
-
- Beedham, R. J., P. C. B. Turnbull, and E. D. Williamson. 2001. Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine 19:4409-4416. - PubMed
-
- Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. T. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. - PubMed
-
- Bull, J. J., and C. R. Parrish. 2002. A binding contract for anthrax. Science 297:201-202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

