Variable tick protein in two genomic groups of the relapsing fever spirochete Borrelia hermsii in western North America
- PMID: 16177341
- PMCID: PMC1230938
- DOI: 10.1128/IAI.73.10.6647-6658.2005
Variable tick protein in two genomic groups of the relapsing fever spirochete Borrelia hermsii in western North America
Abstract
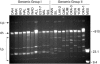
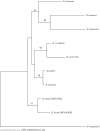
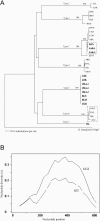
Borrelia hermsii is the primary cause of tick-borne relapsing fever in North America. When its tick vector, Ornithodoros hermsi, acquires these spirochetes from the blood of an infected mammal, the bacteria switch their outer surface from one of many bloodstream variable major proteins (Vmps) to a unique protein, Vtp (Vsp33). Vtp may be critical for successful tick transmission of B. hermsii; however, the gene encoding this protein has been described previously in only one isolate. Here we identified and sequenced the vtp gene in 31 isolates of B. hermsii collected over 40 years from localities throughout much of its known geographic distribution. Seven major Vtp types were found. Little or no sequence variation existed within types, but between them significant variation was observed, similar to the pattern of diversity described for the outer surface protein C (OspC) gene in Lyme disease spirochetes. The pattern of sequence relatedness among the Vtp types was incongruent in two branches compared to two genomic groups identified among the isolates by multilocus sequence typing of the 16S rRNA, flaB, gyrB, and glpQ genes. Therefore, both horizontal transfer and recombination within and between the two genomic groups were responsible for some of the variation observed in the vtp gene. O. hermsi ticks were capable of transmitting spirochetes in the newly identified genomic group. Therefore, given the longevity of the tick vector and persistent infection of spirochetes in ticks, these arthropods rather than mammals may be the likely host where the exchange of spirochetal DNA occurs.
Figures

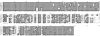

References
-
- Bacon, R. M., M. A. Pilgard, B. J. B. Johnson, S. J. Raffel, and T. G. Schwan. 2004. Glycerophosphodiester phosphodiesterase gene (glpQ) of Borrelia lonestari identified as a target for differentiating Borrelia species associated with hard ticks (Acari: Ixodidae). J. Clin. Microbiol. 42:2326-2328. - PMC - PubMed
-
- Barbour, A. G. 2003. Antigenic variation in Borrelia: relapsing fever and Lyme borreliosis, p. 319-356. In A. Craig, and A. Scherf (ed.), Antigenic variation. Academic Press, Ltd., London, England.
-
- Barbour, A. G. 1987. Immunobiology of relapsing fever, p. 125-137. In J. M. Cruse and R. E. Lewis, Jr. (ed.), Contributions to microbiology and immunology, vol. 8. Karger, Basel, Switzerland. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

