Modulation of flexion reflex induced by hip angle changes in human spinal cord injury
- PMID: 16177832
- PMCID: PMC1361117
- DOI: 10.1007/s00221-005-0112-0
Modulation of flexion reflex induced by hip angle changes in human spinal cord injury
Abstract
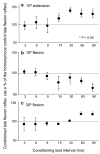
The flexion reflex can be elicited via stimulation of skin, muscle, and high-threshold afferents inducing a generalized flexion of the limb. In spinalized animal models this reflex is quite prominent and is strongly modulated by actions of hip proprioceptors. However, analogous actions on the flexion reflex in spinal cord injured (SCI) humans have not yet been examined. In this study, we investigated the effects of imposed static hip angle changes on the flexion reflex in ten motor incomplete SCI subjects when input from plantar cutaneous mechanoreceptors was also present. Flexion reflexes were elicited by low-intensity stimulation of the sural nerve at the lateral malleolus, and were recorded from the ipsilateral tibialis anterior (TA) muscle. Plantar skin stimulation was delivered through two surface electrodes placed on the metatarsals, and was initiated at different delays ranging from 3 to 90 ms. We found that non-noxious sural nerve stimulation induced two types of flexion reflexes in the TA muscle, an early, and a late response. The first was observed only in three subjects and even in these subjects, it appeared irregularly. In contrast, the second (late) flexion reflex was present uniformly in all ten subjects and was significantly modulated during hip angle changes. Flexion reflexes recorded with hip positioned at different angles were compared to the associated control reflexes recorded with hip flexed at 10 degrees. Hip flexion (30 degrees, 40 degrees) depressed the late flexion reflex, while no significant effects were observed with the hip set in neutral angle (0 degrees). Strong facilitatory effects on the late flexion reflex were observed with the hip extended to 10 degrees. Moreover, the effects of plantar skin stimulation on the flexion reflex were also found to depend on the hip angle. The results suggest that hip proprioceptors and plantar cutaneous mechanoreceptors strongly modulate flexion reflex pathways in chronic human SCI, verifying that this type of sensory afferent feedback interact with spinal interneuronal circuits that have been considered as forerunners of stepping and locomotion. The sensory consequences of this afferent input should be considered in rehabilitation programs aimed to restore movement and sensorimotor function in these patients.
Figures
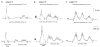
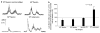
References
-
- Anden NE, Jukes MG, Lundberg A, Vyklicky L. The effect of Dopa on the spinal cord 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966a;67:373–386. - PubMed
-
- Anden NE, Jukes MG, Lundberg A. The effect of Dopa on the spinal cord. 2. A pharmacological analysis. Acta Physiol Scand. 1966b;67:387–397. - PubMed
-
- Anden NE, Jukes MG, Lundberg A, Vyklicky L. The effect of Dopa on the spinal cord. 3. Depolarization evoked in the central terminals of ipsilateral Ia afferents by volleys in the FRA. Acta Physiol Scand. 1966c;68:322–336.
-
- Andersen OK, Sonnenborg FA, Arendt-Nielsen L. Modular organization of human leg withdrawal reflexes elicited by electrical stimulation of the foot sole. Muscle Nerve. 1999;22:1520–1530. - PubMed
-
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practioner. 1964;192:540–542. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

