The paradox of matrix metalloproteinases in infectious disease
- PMID: 16178851
- PMCID: PMC1809491
- DOI: 10.1111/j.1365-2249.2005.02840.x
The paradox of matrix metalloproteinases in infectious disease
Abstract
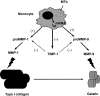
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that perform multiple roles in the normal immune response to infection. MMPs facilitate leucocyte recruitment, cytokine and chemokine processing, defensin activation and matrix remodelling. However, excess MMP activity following infection may lead to immunopathology that causes host morbidity or mortality and favours pathogen dissemination or persistence. Here, we review the normal functions of MMPs in immunity and then discuss viral and bacterial infections where excess MMP activity has been implicated in pathology, specifically examining HIV, HTLV-1, hepatitis B, endotoxin shock, Helicobacter pylori and Mycobacterium tuberculosis. Tissue destruction may be exacerbated further by bacterial-derived enzymes which activate the host pro-MMPs. Finally, the potential for therapeutic targeting of excess MMP activity in infection is considered.
Figures

References
-
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. - PubMed
-
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–14. - PubMed
-
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

