GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males
- PMID: 16178862
- PMCID: PMC1809497
- DOI: 10.1111/j.1365-2249.2005.02904.x
GnRH-I and GnRH-II have differential modulatory effects on human peripheral blood mononuclear cell proliferation and interleukin-2 receptor gamma-chain mRNA expression in healthy males
Abstract
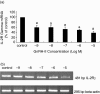
GnRH-I and its receptor (GnRHR-I) have previously been demonstrated and shown to be biologically active in the immune system, notably within peripheral lymphocytes. Recently however, a second form of GnRH (GnRH-II) has been described in the human. The functions of both these neuropeptides in PMBCs have not been understood yet. The present study was therefore designed to investigate the effects of GnRH-I and/or GnRH-II on human PMBC proliferation in males. Secondly, the effects of GnRH-I and GnRH-II on IL-2 dependent lymphocyte proliferation were examined. Finally, we analysed the role of GnRH-I and GnRH-II in IL-2R gamma-chain expression. Peripheral venous blood samples were obtained from six male healthy volunteers (Mean age 27.75 +/- 1.5). Non-radioactive cell proliferation assay was used for proliferation studies and we used quantitative real-time RT-PCR to examine the role of GnRH-I and GnRH-II on IL-2R gamma-chain expression in PMBCs. Treatment of PMBCs with GnRH-I (10(-9) M and 10(-5) M) and with interleukin-2 (IL-2) (50 U/ml) resulted in a significant increase in cell proliferation compared with the untreated control. PMBCs cotreated with IL-2 and GnRH-I demonstrated higher proliferative responses than IL-2 treatment alone, the enhancement of GnRH-I on IL-2 response being significant only at GnRH-I concentration of 10(-5) M. Co-incubation of IL-2+ GnRH 10(-5) M with a GnRH antagonist (Cetrorelix; 10(-6) M) significantly decreased the proliferation. GnRH-II did not affect the proliferation of PMBCs alone, and did not alter the proliferative response to IL-2. The proliferative responses to GnRH-I (alone and with IL-2) were significantly attenuated by GnRH-II coincubation (each in equal molar concentrations; 10(-9) M to 10(-5) M). It was found that GnRH-I increased the expression of IL-2Rgamma mRNA in a dose dependent manner, with a significant increase of percentage 162.3 +/- 14 of control at 10(-5) M. In contrast, IL-2Rgamma expression was significantly decreased in all concentrations of GnRH-II (10(-9) M to10(-5) M), and the maximum decrease was detected at 10(-5) M, with percentage 37.7 +/- 6.6 of control. All these findings strongly suggest that regulation of IL-2R expression may therefore be an important target for GnRH-I and GnRH-II in PMBCs in males. In summary, present study clearly demonstrates the differential effects of GnRH-I and GnRH-II on PMBC proliferation, IL-2 proliferative response, and IL-2Rgamma expression in PMBCs in males. To our knowledge, our observations provide the first evidence for the interactions of these local neuropeptides at lymphocyte level. Further experimental data in human are warranted to explore the clinical implications of these data.
Figures

Similar articles
-
Expression of gonadotropin-releasing hormone type-I (GnRH-I) and type-II (GnRH-II) in human peripheral blood mononuclear cells (PMBCs) and regulation of B-lymphoblastoid cell proliferation by GnRH-I and GnRH-II.Exp Clin Endocrinol Diabetes. 2004 Nov;112(10):587-94. doi: 10.1055/s-2004-830404. Exp Clin Endocrinol Diabetes. 2004. PMID: 15578334
-
Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro.J Clin Endocrinol Metab. 1999 Feb;84(2):743-50. doi: 10.1210/jcem.84.2.5440. J Clin Endocrinol Metab. 1999. PMID: 10022447
-
Cetrorelix, a gonadotropin-releasing hormone antagonist, induces the expression of melatonin receptor 1a in the gonadotropin-releasing hormone neuronal cell line GT1-7.Neuroendocrinology. 2009;90(3):251-9. doi: 10.1159/000231993. Epub 2009 Jul 30. Neuroendocrinology. 2009. PMID: 19641296
-
GnRH-II mRNA expression in tumor tissue and peripheral blood mononuclear cells (PBMCs) in patients with malignant and benign ovarian tumors.Eur J Obstet Gynecol Reprod Biol. 2010 Mar;149(1):92-6. doi: 10.1016/j.ejogrb.2009.11.009. Epub 2009 Dec 16. Eur J Obstet Gynecol Reprod Biol. 2010. PMID: 20018426
-
Differential regulation of gonadotropin-releasing hormone (GnRH)I and GnRHII messenger ribonucleic acid by gonadal steroids in human granulosa luteal cells.J Clin Endocrinol Metab. 2003 Feb;88(2):663-72. doi: 10.1210/jc.2002-020866. J Clin Endocrinol Metab. 2003. PMID: 12574197
Cited by
-
Androgen deprivation therapy and cardiovascular disease: what is the linking mechanism?Ther Adv Urol. 2016 Apr;8(2):118-29. doi: 10.1177/1756287215617872. Epub 2015 Nov 30. Ther Adv Urol. 2016. PMID: 27034724 Free PMC article. Review.
-
Gonadotropin-releasing hormone stimulates biliary proliferation by paracrine/autocrine mechanisms.Am J Pathol. 2015 Apr;185(4):1061-72. doi: 10.1016/j.ajpath.2014.12.004. Am J Pathol. 2015. PMID: 25794706 Free PMC article.
-
Degarelix versus luteinizing hormone-releasing hormone agonists for the treatment of prostate cancer.Expert Opin Pharmacother. 2017 Jun;18(8):825-832. doi: 10.1080/14656566.2017.1328056. Epub 2017 May 19. Expert Opin Pharmacother. 2017. PMID: 28480768 Free PMC article. Review.
-
A meta-analysis of cardiovascular events in intermittent androgen-deprivation therapy versus continuous androgen-deprivation therapy for prostate cancer patients.Prostate Cancer Prostatic Dis. 2016 Dec;19(4):333-339. doi: 10.1038/pcan.2016.35. Epub 2016 Sep 6. Prostate Cancer Prostatic Dis. 2016. PMID: 27595915 Review.
-
Assessment and Mitigation of Cardiovascular Risk for Prostate Cancer Patients: A Review of the Evidence.Int J Clin Pract. 2022 May 17;2022:2976811. doi: 10.1155/2022/2976811. eCollection 2022. Int J Clin Pract. 2022. PMID: 35685515 Free PMC article. Review.
References
-
- Conn PM, Crowley WF. Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;324:93–103. - PubMed
-
- Whitelaw PF, Eidne KA, Sellar R, Smyth CD, Hillier SG. Gonadotropin-releasing hormone receptor messenger ribonucleic acid expression in rat ovary. Endocrinology. 1995;136:172–9. - PubMed
-
- Kakar SS, Musgrove LC, Devor DC, Sellers JC, Neill JD. Cloning, sequencing, and expression of human gonadotropin releasing hormone (GnRH) receptor. Biochem Biophys Res Commun. 1992;189:289–95. - PubMed
-
- Standaert FE, Chew BP, De Avila D, Reeves JJ. Presence of luteinizing hormone-releasing hormone binding sites in cultured porcine lymphocytes. Biol Reprod. 1992;46:997–1000. - PubMed
-
- Weesner GD, Becker BA, Matteri RL. Expression of luteinizing hormone-releasing hormone and its receptor in porcine immune tissues. Life Sci. 1997;61:1643–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

