Detection of apoptosis-specific autoantibodies directed against granzyme B-induced cleavage fragments of the SS-B (La) autoantigen in sera from patients with primary Sjögren's syndrome
- PMID: 16178869
- PMCID: PMC1809481
- DOI: 10.1111/j.1365-2249.2005.02888.x
Detection of apoptosis-specific autoantibodies directed against granzyme B-induced cleavage fragments of the SS-B (La) autoantigen in sera from patients with primary Sjögren's syndrome
Abstract
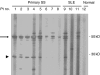
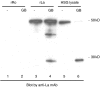
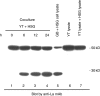
The objective of this study was to detect autoantibodies against granzyme B cleavage products in sera from patients with primary Sjögren's syndrome (SS). Cell lysates derived from human salivary gland (HSG) cell lines were incubated with granzyme B. The susceptibility to the generation of cleavage fragments of SS autoantigens was assayed by immunoblotting using sera from 57 primary SS patients, 17 primary SS patients with malignant lymphoma (ML), 28 systemic lupus erythematosus (SLE) patients, and 20 healthy controls. A 27 kD protein was recognized by serum autoantibodies in 8 (14.0%) of 57 primary SS patients, 5 (29.4%) of 17 SS patients with ML, 2 (7.1%) of 28 SLE patients, but not in 20 normal subjects. This protein was recognized by anti-SSB (La) monoclonal antibodies. Granzyme B-treated recombinant La protein was also shown to migrate as a discrete 27 kD protein by SDS PAGE. Blocking studies demonstrated the existence of an apoptosis-specific B cell epitope present in sera from 2 of 8 primary SS patients and in 2 of 5 primary SS patients with ML which recognized the 27 kD protein. Granzyme B-induced La fragments are generated during cytotoxicity in vitro. This is the first report describing autoantibodies in sera from primary SS patients that specifically recognize fragments of the La protein that are produced by the granzyme B protease.
Figures

Similar articles
-
Serum antibodies from patients with primary Sjögren's syndrome and systemic lupus erythematosus recognize multiple epitopes on the La(SS-B) autoantigen resembling viral protein sequences.Scand J Immunol. 1996 Jan;43(1):115-21. doi: 10.1046/j.1365-3083.1996.d01-2.x. Scand J Immunol. 1996. PMID: 8560190
-
Clinical, immunological, and immunogenetic aspects of autoantibody production against Ro/SSA, La/SSB and their linear epitopes in primary Sjögren's syndrome (pSS): a European multicentre study.Ann Rheum Dis. 2002 May;61(5):398-404. doi: 10.1136/ard.61.5.398. Ann Rheum Dis. 2002. PMID: 11959762 Free PMC article.
-
Can B cell epitopes of 60 kDa Ro distinguish systemic lupus erythematosus from Sjögren's syndrome?Lupus. 2001;10(8):547-53. doi: 10.1191/096120301701549679. Lupus. 2001. PMID: 11530996
-
Fine specificity of the autoimmune response to the Ro/SSA and La/SSB ribonucleoproteins.Arthritis Rheum. 1999 Feb;42(2):199-209. doi: 10.1002/1529-0131(199902)42:2<199::AID-ANR1>3.0.CO;2-1. Arthritis Rheum. 1999. PMID: 10025913 Review.
-
Autoantibodies and their target antigens in Sjögren's syndrome.Neth J Med. 1992 Apr;40(3-4):140-7. Neth J Med. 1992. PMID: 1603204 Review.
Cited by
-
Granzyme serine proteases in inflammation and rheumatic diseases.Nat Rev Rheumatol. 2024 Jun;20(6):361-376. doi: 10.1038/s41584-024-01109-5. Epub 2024 Apr 30. Nat Rev Rheumatol. 2024. PMID: 38689140 Review.
-
Non-mutational neoantigens in disease.Nat Immunol. 2024 Jan;25(1):29-40. doi: 10.1038/s41590-023-01664-1. Epub 2024 Jan 2. Nat Immunol. 2024. PMID: 38168954 Free PMC article. Review.
-
Modulation of Apoptosis by Cytotoxic Mediators and Cell-Survival Molecules in Sjögren's Syndrome.Int J Mol Sci. 2018 Aug 11;19(8):2369. doi: 10.3390/ijms19082369. Int J Mol Sci. 2018. PMID: 30103522 Free PMC article. Review.
-
Autoantigen discovery with a synthetic human peptidome.Nat Biotechnol. 2011 May 22;29(6):535-41. doi: 10.1038/nbt.1856. Nat Biotechnol. 2011. PMID: 21602805 Free PMC article.
-
General Approach for Tetramer-Based Identification of Autoantigen-Reactive B Cells: Characterization of La- and snRNP-Reactive B Cells in Autoimmune BXD2 Mice.J Immunol. 2015 May 15;194(10):5022-34. doi: 10.4049/jimmunol.1402335. Epub 2015 Apr 17. J Immunol. 2015. PMID: 25888644 Free PMC article.
References
-
- Moutsopoulos HM, Chused TM, Mann DL, et al. Sjogren's syndrome (Sicca syndrome): current issues. Ann Intern Med. 1980;92:212–26. - PubMed
-
- Tengner P, Halse AK, Haga HJ, Jonsson R, Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjogren's syndrome. Arthritis Rheum. 1998;41:2238–48. - PubMed
-
- Utz PJ, Anderson P. Posttranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens. Arthritis Rheum. 1998;41:1152–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

